Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
RESEARCH PAPER
Epidemiological characteristics of treated multiple sclerosis patients – Lublin experience (South-Eastern Poland)
1
Department of Neurology, Students’ Scientific Association of Neurology, Medical University, Lublin, Poland
2
Department of Neurology, Medical University, Lublin, Poland
3
Department of Neurology, Provincial Specialist Hospital, Lublin, Poland
Corresponding author
Ann Agric Environ Med. 2025;32(4):566-571
KEYWORDS
risk factorsepidemiologyincidencemorbidityprevalence ratemultiple sclerosisMS treatmentmodestly effective drugsmoderately effective drugshighly effective drugs
TOPICS
ABSTRACT
Introduction and objective:
Multiple sclerosis (MS) is one of the most common neurological diseases affecting about 2.8 million people worldwide. The aim of this study is to analyze the current MS patient population in Lublin, south-east Poland, to enhance understanding of disease risk and treatment options.
Material and methods:
The study was conducted in 2023–2024 in the hospitals and neurological MS centres in the city of Lublin. Demographic and clinical data of adult patients with MS were collected from registries. The obtained results were compared with the results of previous studies, including a study conducted in Lublin in 2004.
Results:
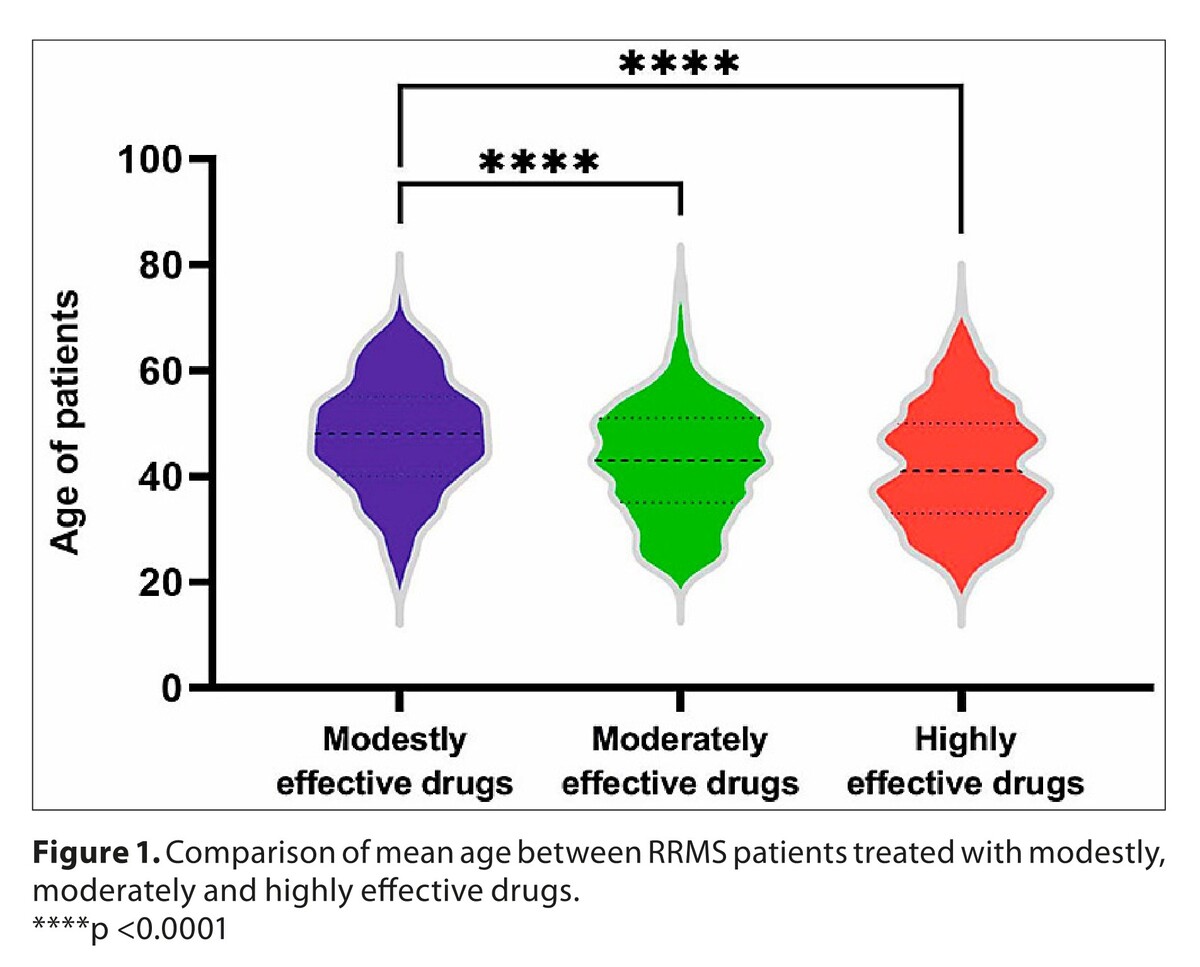
In Lublin, there are 1034 patients treated because of MS, approximately 70% are women. The mean age of patients is 46.25 ±12.11 years. About 79.2% have a relapsing-remitting (RR) type of MS, 8.0% – secondary progressive (SP) type, 11.4% – primary progressive (PP) type, and 1.4%- rapidly evolving or severe type (RES). About 28% of RRMS patients are treated with modestly effective drugs (i.e. platform therapy), with mean disability assessed by the Expanded Disability Status Scale, EDSS=2.11, about 40% of these patients- with moderately effective drugs, with mean EDSS=1.99, and about 28% of RRMS patients with highly effective drugs (HED), with mean EDSS=2.53.
Conclusions:
Compared to 2004, when 204 patients were recorded, the number of MS patients in Lublin has increased fivefold due to improved diagnosis, treatment, and rising MS incidence. Mean EDSS is higher in RRMS patients treated with HED compared to patients treated with moderately effective drugs, probably due to higher activity of MS and more rapid progression of disability in patients requiring more effective therapy. Further research is needed to deepen understanding of MS and its treatment.
Multiple sclerosis (MS) is one of the most common neurological diseases affecting about 2.8 million people worldwide. The aim of this study is to analyze the current MS patient population in Lublin, south-east Poland, to enhance understanding of disease risk and treatment options.
Material and methods:
The study was conducted in 2023–2024 in the hospitals and neurological MS centres in the city of Lublin. Demographic and clinical data of adult patients with MS were collected from registries. The obtained results were compared with the results of previous studies, including a study conducted in Lublin in 2004.
Results:
In Lublin, there are 1034 patients treated because of MS, approximately 70% are women. The mean age of patients is 46.25 ±12.11 years. About 79.2% have a relapsing-remitting (RR) type of MS, 8.0% – secondary progressive (SP) type, 11.4% – primary progressive (PP) type, and 1.4%- rapidly evolving or severe type (RES). About 28% of RRMS patients are treated with modestly effective drugs (i.e. platform therapy), with mean disability assessed by the Expanded Disability Status Scale, EDSS=2.11, about 40% of these patients- with moderately effective drugs, with mean EDSS=1.99, and about 28% of RRMS patients with highly effective drugs (HED), with mean EDSS=2.53.
Conclusions:
Compared to 2004, when 204 patients were recorded, the number of MS patients in Lublin has increased fivefold due to improved diagnosis, treatment, and rising MS incidence. Mean EDSS is higher in RRMS patients treated with HED compared to patients treated with moderately effective drugs, probably due to higher activity of MS and more rapid progression of disability in patients requiring more effective therapy. Further research is needed to deepen understanding of MS and its treatment.
REFERENCES (25)
1.
Dendrou CA, Fugger L, Friese MA. Immunopathology of multiple sclerosis. Nat Rev Immunol. 2015;15(9):545–558. https://doi:10.1038/nri3871.
2.
Travers BS, Tsang BK, Barton JL. Multiple sclerosis: Diagnosis, disease-modifying therapy and prognosis. Aust J Gen Pract. 2022;51(4):199–206. https://doi:10.31128/AJGP-07-2....
3.
Huisman E, Papadimitropoulou K, Jarrett J, et al. Systematic literature review and network meta-analysis in highly active relapsing-remitting multiple sclerosis and rapidly evolving severe multiple sclerosis. BMJ Open. 2017;7(3):e013430. https://doi:10.1136/bmjopen-20....
4.
Thompson AJ, Baranzini SE, Geurts J, et al. Multiple sclerosis. Lancet. 2018;391(10130):1622–1636. https://doi:10.1016/S0140-6736....
5.
Bjornevik K, Cortese M, Healy BC, et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science. 2022;375(6578):296–301. https://doi:10.1126/science.ab....
6.
Rosiak K, Zagożdżon P. Environmental factors in epidemiology of multiple sclerosis. Probl Hig Epidemiol. 2012;93(4):627–631. https://doi.org/10.12775/JEHS.....
7.
Manouchehrinia A, Huang J, Hillert J, et al. Smoking Attributable Risk in Multiple Sclerosis. Front Immunol. 2022;13:840158. https://doi:10.3389/fimmu.2022....
8.
Suliga E, Cieśla E, Jasińska E, et al. Lifestyle and health of individuals with multiple sclerosis according to body mass index: initial results. Medical Studies. 2022;38(2):140–151. https://doi:10.5114/ms.2022.11....
9.
Hauser SL, Cree BAC. Treatment of Multiple Sclerosis: A Review. Am J Med. 2020;133(12):1380–1390.e2. https://doi:10.1016/j.amjmed.2....
10.
Kułakowska A, Mirowska-Guzel D, Kalinowska A, et al. Leczenie modyfikujące przebieg stwardnienia rozsianego. Rekomendacje Sekcji Stwardnienia Rozsianego i Neuroimmunologii Polskiego Towarzystwa Neurologicznego. Pol Przegl Neurol. 2023;19(3):163–189. https://doi:10.5603/PPN.a2023.....
11.
Drug programme B.29 reimbursed by the Ministry of Health in Poland https://ptsr.org.pl/strona/64,... (access: 2024.07.20).
12.
Kappos L, Bar-Or A, Cree BAC, et al. Siponimod versus placebo in secondary progressive multiple sclerosis (EXPAND): a double-blind, randomised, phase 3 study. Lancet. 2018;391(10127):1263–1273. https://doi:10.1016/S0140-6736....
13.
Walton C, King R, Rechtman L, et al. Rising prevalence of multiple sclerosis worldwide: Insights from the Atlas of MS, third edition. Mult Scler. 2020;26(14):1816–1821. https://doi:10.1177/1352458520....
14.
Multiple sclerosis prevalance by the Ministry of Health in Poland https://analizy.mz.gov.pl/html... (access: 2024.05.10).
15.
Łobińska A, Stelmasiak Z. Wybrane epidemiologiczne aspekty stwardnienia rozsianego w populacji miasta Lublina. Neurol Neurochirurgia Pol. 2004;38:361–366.
16.
Filippi M, Preziosa P, Meani A, et al. Performance of the 2017 and 2010 Revised McDonald Criteria in Predicting MS Diagnosis After a Clinically Isolated Syndrome: A MAGNIMS Study. Neurol. 2022;98(1):e1-e14. https://doi:10.1212/WNL.000000....
17.
Rejdak K, Stelmasiak Z, Grieb P. Cladribine induces long lasting oligoclonal bands disappearance in relapsing multiple sclerosis patients: 10-year observational study. Mult Scler Relat Disord. 2019;27:117–120. https://doi:10.1016/j.msard.20....
18.
Wnuk M, Maluchnik M, Perwieniec J, et al. Multiple sclerosis incidence and prevalence in Poland: Data from administrative health claims. Mult Scler Relat Disord. 2021;55:103162. https://doi:10.1016/j.msard.20....
19.
Wiendl H, Gold R, Berger T, et al. Multiple Sclerosis Therapy Consensus Group (MSTCG): position statement on disease-modifying therapies for multiple sclerosis (white paper). Ther Adv Neurol Disord. 2021;14:17562864211039648. https://doi:10.1177/1756286421....
20.
Hellwig K, Rog D, McGuigan C, et al. Interim Analysis of Pregnancy Outcomes After Exposure to Dimethyl Fumarate in a Prospective International Registry. Neurol Neuroimmunol Neuroinflamm. 2021;9(1):e1114. https://doi:10.1212/NXI.000000....
21.
Hauser SL, Zielman R, Das Gupta A, et al. Efficacy and safety of four-year ofatumumab treatment in relapsing multiple sclerosis: The ALITHIOS open-label extension. Mult Scler. 2023;29(11–12):1452–1464. https://doi:10.1177/1352458523....
22.
Kern DM, Cepeda MS. Treatment patterns and comorbid burden of patients newly diagnosed with multiple sclerosis in the United States. BMC Neurol. 2020;20(1):296. https://doi:10.1186/s12883-020....
23.
Holstiege J, Akmatov MK, Klimke K, et al. Trends in administrative prevalence of multiple sclerosis and utilization patterns of disease modifying drugs in Germany. Mult Scler Relat Disord. 2022;59:103534. https://doi:10.1016/j.msard.20....
24.
Hsu CY, Ro LS, Chen LJ, et al. Epidemiology, treatment patterns and healthcare utilizations in multiple sclerosis in Taiwan. Sci Rep. 2021;11: 7727. https://doi.org/10.1038/s41598....
25.
Corboy JR, Fox RJ, Kister I, et al. Risk of new disease activity in patients with multiple sclerosis who continue or discontinue disease-modifying therapies (DISCOMS): a multicentre, randomised, single-blind, phase 4, non-inferiority trial. Lancet Neurol. 2023;22(7):568–577. https://doi:10.1016/S1474-4422....
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.