Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
RESEARCH PAPER
Comparative analysis of bacterial microbiomes in Ixodes ricinus female ticks from military training areas in Poland
1
Biodefence Laboratory, Biomedical Engineering Centre, Institute of Optoelectronics, Military University of Technology, Warsaw, Poland
2
Faculty of Physical Education, Józef Piłsudski University of Physical Education, Warsaw, Poland
3
Preventive Medicine Division, Epidemiological Response Centre of The Polish Armed Forces, Warsaw, Poland
4
Polish Armed Forces, Military Preventive Medicine Centre, Gdynia, Poland
5
Laboratory of Genomics and Bioinformatics, Hirszfeld Institute of Immunology and Experimental Therapy, Polish Academy of Sciences, Wrocław, Poland
6
Department of Molecular Biology, Military Institute of Aviation Medicine, Warsaw, Poland
Corresponding author
Katarzyna Komar
Department of Molecular Biology, Military Institute of Aviation Medicine, Warsaw, Poland
Department of Molecular Biology, Military Institute of Aviation Medicine, Warsaw, Poland
KEYWORDS
epidemiologyBorreliaIxodes ricinusRickettsiaOne Healthnext-generation sequencingtick-borne pathogensTick microbiomeEhrlichiamilitary areas
TOPICS
ABSTRACT
Introduction and objective:
Ticks, especially Ixodes ricinus, are known vectors of multiple pathogens affecting human and animal health. Monitoring tick microbiomes, particularly in areas of military activity, is essential to understand the epidemiological threats they pose. This study investigates the microbiomes of I. ricinus ticks collected from military areas in Poland using next-generation sequencing (NGS).
Material and methods:
Ixodes ricinus ticks were collected in spring and autumn from military training grounds using the flagging method. After segregation (by stage and gender), DNA was isolated, libraries were prepared, and sequencing was performer. Data quality was assessed with fastQC. Pathogens were identified using Kraken2. The data was further analyzed using Bracken’s classification methodology
Results:
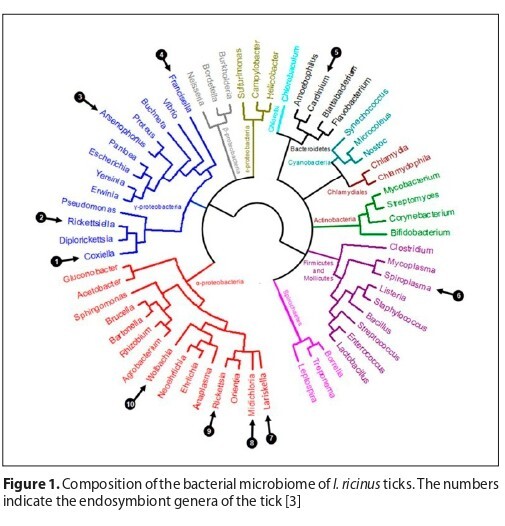
Metagenomic analysis of Ixodes ricinus ticks revealed a diverse bacterial community composed of symbionts, environmental taxa, and potential pathogens. Core endosymbionts were consistently detected across all samples, while medically relevant genera, such as Borrelia, Rickettsia, Ehrlichia and Bartonella, were also identified. The results highlight both the complexity of the tick microbiome and its potential importance for human and animal health.
Conclusions:
The study provides a preliminary overview of the microbiome of adult Ixodes ricinus ticks from Polish military training areas. Core endosymbionts were consistently detected, while variation in less abundant taxa suggests environmental influences. The presence of potential pathogens highlights the need for broader studies, and underlines the relevance of metagenomic approaches for public health and military medicine.
Ticks, especially Ixodes ricinus, are known vectors of multiple pathogens affecting human and animal health. Monitoring tick microbiomes, particularly in areas of military activity, is essential to understand the epidemiological threats they pose. This study investigates the microbiomes of I. ricinus ticks collected from military areas in Poland using next-generation sequencing (NGS).
Material and methods:
Ixodes ricinus ticks were collected in spring and autumn from military training grounds using the flagging method. After segregation (by stage and gender), DNA was isolated, libraries were prepared, and sequencing was performer. Data quality was assessed with fastQC. Pathogens were identified using Kraken2. The data was further analyzed using Bracken’s classification methodology
Results:
Metagenomic analysis of Ixodes ricinus ticks revealed a diverse bacterial community composed of symbionts, environmental taxa, and potential pathogens. Core endosymbionts were consistently detected across all samples, while medically relevant genera, such as Borrelia, Rickettsia, Ehrlichia and Bartonella, were also identified. The results highlight both the complexity of the tick microbiome and its potential importance for human and animal health.
Conclusions:
The study provides a preliminary overview of the microbiome of adult Ixodes ricinus ticks from Polish military training areas. Core endosymbionts were consistently detected, while variation in less abundant taxa suggests environmental influences. The presence of potential pathogens highlights the need for broader studies, and underlines the relevance of metagenomic approaches for public health and military medicine.
REFERENCES (18)
1.
Perumalsamy N, Sharma R, Subramanian M, et al. Hard Ticks as Vectors: The Emerging Threat of Tick-Borne Diseases in India. Pathogens. 2024 Jul 2;13(7):556. doi:10.3390/pathogens13070556. PMID: 39057783; PMCID: PMC11279560.
2.
Dennis DT, Piesman JF. Overview of tick-borne infections of humans. In: Goodman JL, Dennis DT, Sonenshine DE, editors. Tick-Borne Diseases of Humans. Washington (DC): ASM Press; 2005. p. 3–11.
3.
Bonnet SI, Binetruy F, Hernández-Jarguín AM, et al. The Tick Microbiome: Why Non-pathogenic Microorganisms Matter in Tick Biology and Pathogen Transmission. Front Cell Infect Microbiol. 2017;7:236. doi:10.3389/fcimb.2017.00236. https://doi.org/10.3389/fcimb.....
4.
Liu MC, Zhang JT, Chen JJ, et al. A global dataset of microbial community in ticks from metagenome study. Sci Data. 2022;9(560). https://doi.org/10.1038/s41597....
5.
Berg G, Rybakova D, Fischer D, et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome. 2020;8(1):103. https://doi.org/10.1186/s40168....
6.
Ahantarig A, Trinachartvanit W, Baimai V, et al. Hard ticks and their bacterial endosymbionts (or would be pathogens). Folia Microbiol (Praha). 2013;58(5):419–428. https://doi.org/10.1007/s12223....
7.
Andreotti R, Perez de Leon AA, Dowd SE, et al. Assessment of bacterial diversity in the cattle tick Rhipicephalus (Boophilus) microplus through tag-encoded pyrosequencing. BMC Microbiol. 2011;11(6). https://doi.org/10.1186/1471-2....
8.
Wu-Chuang A, Hodžić A, Mateos-Hernández L, et al. Current debates and advances in tick microbiome research. Curr Res Parasitol Vector Borne Dis. 2021;1:100036. doi:10.1016/j.crpvbd.2021.100036.
9.
Landesman WJ, Mulder K, Page Fredericks L, et al. Cross-kingdom analysis of nymphal-stage Ixodes scapularis microbial communities in relation to Borrelia burgdorferi infection and load. FEMS Microbiol Ecol. 2019;95(12):fiz167. https://doi.org/10.1093/femsec....
10.
Claerebout E, Losson B, Cochez C, et al. Prevalence of Anaplasma phagocytophilum, Borrelia burgdorferi (s.l.) and Babesia species in questing Ixodes ricinus ticks from Belgium. Ticks and Tick-borne Diseases, 2023;14(4), 102174. https://doi.org/10.1016/j.ttbd....
11.
Wu-Chuang A, Hodžić A, Mateos-Hernández L, et al. Anti-Microbiota Vaccines Modulate the Tick Microbiome in a Taxon-Specific Manner. Microbiome. 2023;11:36. https://doi.org/10.1186/s40168....
12.
Bonnet SI, Pollet T. Update on the intricate tango between tick microbiomes and tick-borne pathogens. Parasite Immunol. 2021;43(5):e12813. https://doi.org/10.1111/pim.12....
13.
Wood DE, Lu J, Langmead B. Improved metagenomic analysis with Kraken 2. Genome Biology. 2019;20:257. https://doi.org/10.1186/s13059....
14.
Lu J, Breitwieser FP, Thielen P, et al. Bracken: Estimating species abundance in metagenomics data. PeerJ Computer Sci. 2017;3:e104. https://doi.org/10.7717/peerj-....
15.
Duron O, Binetruy F, Noël V, et al. Tick-bacteria mutualism depends on B vitamin synthesis pathways. Curr Biol. 2018;28(12):1896–1902.e5. https://doi.org/10.1016/j.cub.....
16.
Dunaj J, Kiewra D, Szymanowski M, Mierzejewska EJ. First metagenomic report of Borrelia americana and Borrelia carolinensis in Poland. Front Cell Infect Microbiol. 2021;11:747849. https://doi.org/10.3389/fcimb.....
17.
Greay TL, Gofton AW, Paparini A, et al. Recent insights into the tick microbiome gained through next-generation sequencing. Parasit Vect. 2018;11:12. https://doi.org/10.1186/s13071....
18.
One World One Health. The Manhattan Principles. Avaliable at: https://oneworldonehealth.wcs.... (access: 2025.05.06).
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.