Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
RESEARCH PAPER
MiRNA-21–5p as a biomarker in EBV-associated oropharyngeal cancer
1
Medical University of Lublin, Poland
2
Masovian Specialist Hospital, Poland
Ann Agric Environ Med. 2023;30(1):77-82
KEYWORDS
TOPICS
ABSTRACT
Introduction and objective:
Epstein-Barr virus (EBV) is associated with cancers of the head and neck, including oropharyngeal cancer, which is increasing in incidence, and biomarker studies have potential in diagnostics and therapy. One of the most commonly deregulated microRNAs in cancers is miR-21–5p. It has been implicated in neoplastic transformation related to EBV infection in several investigations. The aim of this study was to determine the level of miR-21–5p in the serum of EBV (+) and EBV (-) oropharyngeal cancer patients.
Material and methods:
The study was carried out on 78 patients with confirmed OPSCC. Statistical analysis was used to investigate the relationship between clinical and demographic characteristics of patients. Enzyme immunoassays were used to determine the levels of miRNA, TLR9 and MMPs and cytokines. Statistical analysis was used to determine the relationship between miR21–5p and TLR9, MMP3, MMP9 levels, and the cytokines studied.
Results:
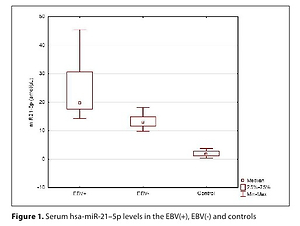
Significantly higher values of all tested parameters for miR-21–5p levels and grading as well as TN stage were found in the EBV (+) group. There was no statistically significant correlation between the miR-21-5p level and the levels of TNFα, VEGF, and TGFβ. Positive correlations were shown between miR-21–5p and IL-10, MMP-3 and -9. There was a negative correlation between the level of miR-21-5p and TLR9.
Conclusions:
The present study showed that in EBV (+) patients the level of miR-21–5p in the serum was significantly higher than in EBV (-) patients. Our study results could influence future strategies for the diagnosis, prevention and treatment of oropharyngeal cancers.
Epstein-Barr virus (EBV) is associated with cancers of the head and neck, including oropharyngeal cancer, which is increasing in incidence, and biomarker studies have potential in diagnostics and therapy. One of the most commonly deregulated microRNAs in cancers is miR-21–5p. It has been implicated in neoplastic transformation related to EBV infection in several investigations. The aim of this study was to determine the level of miR-21–5p in the serum of EBV (+) and EBV (-) oropharyngeal cancer patients.
Material and methods:
The study was carried out on 78 patients with confirmed OPSCC. Statistical analysis was used to investigate the relationship between clinical and demographic characteristics of patients. Enzyme immunoassays were used to determine the levels of miRNA, TLR9 and MMPs and cytokines. Statistical analysis was used to determine the relationship between miR21–5p and TLR9, MMP3, MMP9 levels, and the cytokines studied.
Results:
Significantly higher values of all tested parameters for miR-21–5p levels and grading as well as TN stage were found in the EBV (+) group. There was no statistically significant correlation between the miR-21-5p level and the levels of TNFα, VEGF, and TGFβ. Positive correlations were shown between miR-21–5p and IL-10, MMP-3 and -9. There was a negative correlation between the level of miR-21-5p and TLR9.
Conclusions:
The present study showed that in EBV (+) patients the level of miR-21–5p in the serum was significantly higher than in EBV (-) patients. Our study results could influence future strategies for the diagnosis, prevention and treatment of oropharyngeal cancers.
ACKNOWLEDGEMENTS
This research was funded by a Research Grant from the Medical University of Lublin, Lublin, Poland (DS 233).
REFERENCES (63)
1.
Broccolo F, Ciccarese G, Rossi A, Anselmi L, Drago F, Toniolo A. Human papillomavirus (HPV) and Epstein-Barr virus (EBV) in keratinizing versus non-keratinizing squamous cell carcinoma of the oropharynx. Infect Agent Cancer. 2018;13:32. doi:10.1186/s13027-018-0205-6.
2.
Carpén T, Syrjänen S, Jouhi L, et al. Epstein-Barr virus (EBV) and polyomaviruses are detectable in oropharyngeal cancer and EBV may have prognostic impact. Cancer Immunol Immunother. 2020;69(8):1615–1626. doi:10.1007/s00262-020-02570-3.
3.
Koleśnik M, Dworzańska A, Polz-Dacewicz M. Wirus Epsteina-Barr w wybranych chorobach nowotworowych. Postępy Biochemii. 2020;66(4):385–389. doi:10.18388/pb.2020_364.
4.
Shannon-Lowe C, Rickinson A. The Global Landscape of EBV-Associated Tumors. Frontiers in Oncology. 2019;9. doi:10.3389/fonc.2019.00713.
5.
Drop B, Strycharz-Dudziak M, Kliszczewska E, Polz-Dacewicz M. Coinfection with Epstein-Barr Virus (EBV), Human Papilloma Virus (HPV) and Polyoma BK Virus (BKPyV) in Laryngeal, Oropharyngeal and Oral Cavity Cancer. Int J Mol Sci. 2017;18(12):E2752. doi:10.3390/ijms18122752.
6.
Chow LQM. Head and Neck Cancer. N Engl J Med. 2020;382(1):60–72. doi:10.1056/NEJMra1715715.
7.
Johnson DE, Burtness B, Leemans CR, Lui VWY, Bauman JE, Grandis JR. Head and neck squamous cell carcinoma. Nat Rev Dis Primers. 2020;6(1):1–22. doi:10.1038/s41572-020-00224-3.
8.
Dioguardi M, Caloro GA, Laino L, et al. Circulating miR-21 as a Potential Biomarker for the Diagnosis of Oral Cancer: A Systematic Review with Meta-Analysis. Cancers. 2020;12(4):936. doi:10.3390/cancers12040936.
9.
Diez-Fraile A, De Ceulaer J, Derpoorter C, et al. Tracking the Molecular Fingerprint of Head and Neck Cancer for Recurrence Detection in Liquid Biopsies. Intern J Molecular Sci. 2022;23(5):2403. doi:10.3390/ijms23052403.
10.
Dumache R. Early Diagnosis of Oral Squamous Cell Carcinoma by Salivary microRNAs. Clin Lab. 2017;63(11):1771–1776. doi:10.7754/clin.lab.2017.170607.
11.
Schneider A, Victoria B, Lopez YN, et al. Tissue and serum microRNA profile of oral squamous cell carcinoma patients. Sci Rep. 2018;8(1):675. doi:10.1038/s41598-017-18945-z.
12.
Manikandan M, Deva Magendhra Rao AK, Arunkumar G, et al. Oral squamous cell carcinoma: microRNA expression profiling and integrative analyses for elucidation of tumourigenesis mechanism. Mol Cancer. 2016;15(1):28. doi:10.1186/s12943-016-0512-8.
13.
Koleśnik M, Stępień E, Polz-Dacewicz M. The role of microRNA (miRNA) as a biomarker in HPV and EBV-related cancers. J Pre Clin Clin Res. 2021;15(2):104–110. doi:10.26444/jpccr/138306.
14.
Tiberio P, Callari M, Angeloni V, Daidone MG, Appierto V. Challenges in Using Circulating miRNAs as Cancer Biomarkers. BioMed Research International. 2015;2015:e731479. doi:10.1155/2015/731479.
15.
Cameron JE, Fewell C, Yin Q, et al. Epstein-Barr virus growth/latency III program alters cellular microRNA expression. Virology. 2008;382(2):257–266. doi:10.1016/j.virol.2008.09.018.
16.
Romano G, Veneziano D, Acunzo M, Croce CM. Small non-coding RNA and cancer. Carcinogenesis. 2017;38(5):485–491. doi:10.1093/carcin/bgx026.
17.
Danarto R, Astuti I, Umbas R, Haryana SM. Urine miR-21-5p and miR-200c-3p as potential non-invasive biomarkers in patients with prostate cancer. Turk J Urol. 2020;46(1):26–30. doi:10.5152/tud.2019.19163.
18.
Irimie-Aghiorghiesei AI, Pop-Bica C, Pintea S, et al. Prognostic Value of MiR-21: An Updated Meta-Analysis in Head and Neck Squamous Cell Carcinoma (HNSCC). J Clin Med. 2019;8(12):2041. doi:10.3390/jcm8122041.
19.
Peltanova B, Raudenska M, Masarik M. Effect of tumor microenvironment on pathogenesis of the head and neck squamous cell carcinoma: a systematic review. Molecular Cancer. 2019;18(1):63. doi:10.1186/s12943-019-0983-5.
20.
Larki P, Ahadi A, Zare A, et al. Up-Regulation of miR-21, miR-25, miR-93, and miR-106b in Gastric Cancer. Iranian Biomed J. 2018;22(6):367–373. doi:10.29252/ibj.22.6.367.
21.
Chang SS, Jiang WW, Smith I, et al. MicroRNA alterations in Head and Neck Squamous Cell Carcinoma. Int J Cancer. 2008;123(12):2791–2797. doi:10.1002/ijc.23831.
22.
Lao TD, Quang MT, Le TAH. The Role of hsa-miR-21 and Its Target Genes Involved in Nasopharyngeal Carcinoma. Asian Pac J Cancer Prev. 2021;22(12):4075–4083. doi:10.31557/APJCP.2021.22.12.4075.
23.
Stępień E, Strycharz-Dudziak M, Malm M, Drop B, Polz-Dacewicz M. Serum and Tissue Level of TLR9 in EBV-Associated Oropharyngeal Cancer. Cancers. 2021;13(16):3981. doi:10.3390/cancers13163981.
24.
Sobin LH, Gospodarowicz MK, Wittekind C. TNM Classification of Malignant Tumours. 7th ed. Washington: Wiley-Blackwell; 2009. p.22–45.
25.
Cardesa A, Gale N, Nadal A, Zidor N. Squamous cell carcinoma. World Health Organization Classification of Tumours Pathology and Genetic of Head and Neck Tumours. In Barnes L, Eveson JW, Reichart P, Sidnousky, editors. Lyon, International Agency for Research on Cancer, 2010; 118–121.
26.
Fołtyn S, Strycharz-Dudziak M, Drop B, Boguszewska A, Polz-Dacewicz M. Serum EBV antibodies and LMP-1 in Polish patients with oropharyngeal and laryngeal cancer. Infectious Agents and Cancer. 2017;12(1):31. doi:10.1186/s13027-017-0141-x.
27.
Aghiorghiesei O, Zanoaga O, Raduly L, et al. Dysregulation of miR-21-5p, miR-93-5p, miR-200c-3p and miR-205-5p in Oral Squamous Cell Carcinoma: A Potential Biomarkers Panel? CIMB. 2022;44(4):1754–1767. doi:10.3390/cimb44040121.
28.
Lamperska KM, Kozlowski P, Kolenda T, et al. Unpredictable changes of selected miRNA in expression profile of HNSCC. Cancer Biomarkers. 2016;16(1):55–64. doi:10.3233/CBM-150540.
29.
Zamani S, Sohrabi A, Hosseini SM, Rahnamaye-Farzami M, Akbari A. Deregulation of miR-21 and miR-29a in Cervical Cancer Related to HPV Infection. MicroRNA. 2019;8(2):110–115. doi:10.2174/2211536607666181017124349.
30.
Liu M, Wang W, Chen H, et al. miR-9, miR-21, miR-27b, and miR-34a Expression in HPV16/58/52-Infected Cervical Cancer. BioMed Research International. 2020;2020:e2474235. doi:10.1155/2020/2474235.
31.
Dundar HZ, Aksoy F, Aksoy SA, et al. Overexpression of miR-21 Is Associated With Recurrence in Patients With Hepatitis B Virus–Mediated Hepatocellular Carcinoma Undergoing Liver Transplantation. Transplantation Proceedings. 2019;51(4):1157–1161. doi:10.1016/j.transproceed.2019.01.089.
32.
Yang GD, Huang TJ, Peng LX, et al. Epstein-Barr Virus_Encoded LMP1 Upregulates MicroRNA-21 to Promote the Resistance of Nasopharyngeal Carcinoma Cells to Cisplatin-Induced Apoptosis by Suppressing PDCD4 and Fas-L. PLOS ONE. 2013;8(10):e78355. doi:10.1371/journal.pone.0078355.
33.
Rosato P, Anastasiadou E, Garg N, et al. Differential regulation of miR-21 and miR-146a by Epstein-Barr virus-encoded EBNA2. Leukemia. 2012;26(11):2343–2352. doi:10.1038/leu.2012.108.
34.
Jamali Z, Asl Aminabadi N, Attaran R, Pournagiazar F, Ghertasi Oskouei S, Ahmadpour F. MicroRNAs as prognostic molecular signatures in human head and neck squamous cell carcinoma: A systematic review and meta-analysis. Oral Oncology. 2015;51(4):321–331. doi:10.1016/j.oraloncology.2015.01.008.
35.
Turunen A, Rautava J, Grénman R, Syrjänen K, Syrjänen S. Epstein-Barr virus (EBV)-encoded small RNAs (EBERs) associated with poor prognosis of head and neck carcinomas. Oncotarget. 2017;8(16):27328–27338. doi:10.18632/oncotarget.16033.
36.
Young LS, Dawson CW. Epstein-Barr virus and nasopharyngeal carcinoma. Chin J Cancer. 2014;33(12):581–590. doi:10.5732/cjc.014.10197.
37.
Tsao SW, Tsang CM, Lo KW. Epstein–Barr virus infection and nasopharyngeal carcinoma. Philosophical Transactions of the Royal Society B: Biological Sciences. 2017;372(1732):20160270. doi:10.1098/rstb.2016.0270.
38.
Nakagomi H, Dolcetti R, Bejarano MT, Pisa P, Kiessling R, Masucci MG. The Epstein-Barr virus latent membrane protein-1 (LMP1) induces interleukin-10 production in Burkitt lymphoma lines. Int J Cancer. 1994;57(2):240–244. doi:10.1002/ijc.2910570218.
39.
Eliopoulos AG, Stack M, Dawson CW, et al. Epstein – Barr virus-encoded LMP1 and CD40 mediate IL-6 production in epithelial cells via an NF-?B pathway involving TNF receptor-associated factors. Oncogene. 1997;14(24):2899–2916. doi:10.1038/sj.onc.1201258.
40.
Bani MR, Garofalo A, Scanziani E, Giavazzi R. Effect of Interleukin-1-beta on Metastasis Formation in Different Tumor Systems. JNCI: J National Canc Institute. 1991;83(2):119–123. doi:10.1093/jnci/83.2.119.
41.
Li BY, Mohanraj D, Olson MC, et al. Human Ovarian Epithelial Cancer Cells Cultured in Vitro Express Both Interleukin 1? and ß Genes1. Cancer Res. 1992;52(8):2248–2252.
42.
Murata M. Inflammation and cancer. Environ Health Prev Med. 2018;23(1):50. doi:10.1186/s12199-018-0740-1.
43.
Korniluk A, Koper O, Kemona H, Dymicka-Piekarska V. From inflammation to cancer. Ir J Med Sci. 2017;186(1):57–62. doi:10.1007/s11845-016-1464-0.
44.
Elgui de Oliveira D, Müller-Coan BG, Pagano JS. Viral Carcinogenesis Beyond Malignant Transformation: EBV in the Progression of Human Cancers. Trends Microbiol. 2016;24(8):649–664. doi:10.1016/j.tim.2016.03.008.
45.
Mui UN, Haley CT, Tyring SK. Viral Oncology: Molecular Biology and Pathogenesis. J Clin Med. 2017;6(12):E111. doi:10.3390/jcm6120111.
46.
Yang ZH, Dai Q, Gu YJ, Guo QX, Gong L. Cytokine and chemokine modification by Toll-like receptor polymorphisms is associated with nasopharyngeal carcinoma. Cancer Sci. 2012;103(4):653–658. doi:10.1111/j.1349-7006.2012.02210.x.
47.
Farhat K, Hassen E, Gabbouj S, Bouaouina N, Chouchane L. Interleukin-10 and interferon-gamma gene polymorphisms in patients with nasopharyngeal carcinoma. Int J Immunogenet. 2008;35(3):197–205. doi:10.1111/j.1744-313X.2008.00752.x.
48.
Beck A, Päzolt D, Grabenbauer GG, et al. Expression of cytokine and chemokine genes in Epstein-Barr virus-associated nasopharyngeal carcinoma: comparison with Hodgkin’s disease. J Pathol. 2001;194(2):145–151. doi:10.1002/path.867.
49.
Quintero-Fabián S, Arreola R, Becerril-Villanueva E, et al. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Frontiers in Oncology. 2019;9. Accessed July 12, 2022. https://www.frontiersin.org/ar....
50.
Gonzalez-Avila G, Sommer B, Mendoza-Posada DA, Ramos C, Garcia-Hernandez AA, Falfan-Valencia R. Matrix metalloproteinases participation in the metastatic process and their diagnostic and therapeutic applications in cancer. Crit Rev Oncol/Hematol. 2019;137:57–83. doi:10.1016/j.critrevonc.2019.02.010.
51.
Alaseem A, Alhazzani K, Dondapati P, Alobid S, Bishayee A, Rathinavelu A. Matrix Metalloproteinases: A challenging paradigm of cancer management. Seminars in Cancer Biology. 2019;56:100–115. doi:10.1016/j.semcancer.2017.11.008.
52.
Wang Y, Wang L, Chen C, Chu X. New insights into the regulatory role of microRNA in tumor angiogenesis and clinical implications. Mol Cancer. 2018;17(1):22. doi:10.1186/s12943-018-0766-4.
53.
Noruzi S, Azizian M, Mohammadi R, et al. Micro-RNAs as critical regulators of matrix metalloproteinases in cancer. J Cell Biochem. 2018;119(11):8694–8712. doi:10.1002/jcb.27182.
54.
Zhu Q, Wang Z, Hu Y, et al. miR-21 promotes migration and invasion by the miR-21-PDCD4-AP-1 feedback loop in human hepatocellular carcinoma. Oncol Rep. 2012;27(5):1660–1668. doi:10.3892/or.2012.1682.
55.
Giovannetti E, Funel N, Peters GJ, et al. MicroRNA-21 in pancreatic cancer: correlation with clinical outcome and pharmacologic aspects underlying its role in the modulation of gemcitabine activity. Cancer Res. 2010;70(11):4528–4538. doi:10.1158/0008-5472.CAN-09-4467.
56.
Lan YY, Yeh TH, Lin WH, et al. Epstein-Barr virus Zta upregulates matrix metalloproteinases 3 and 9 that synergistically promote cell invasion in vitro. PLoS One. 2013;8(2):e56121. doi:10.1371/journal.pone.0056121.
57.
Chandan K, Gupta M, Sarwat M. Role of Host and Pathogen-Derived MicroRNAs in Immune Regulation During Infectious and Inflammatory Diseases. Front Immunol. 2020;10:3081. doi:10.3389/fimmu.2019.03081.
58.
Acuña SM, Floeter-Winter LM, Muxel SM. MicroRNAs: Biological Regulators in Pathogen–Host Interactions. Cells. 2020;9(1):113. doi:10.3390/cells9010113.
59.
Ojha CR, Rodriguez M, Dever SM, Mukhopadhyay R, El-Hage N. Mammalian microRNA: an important modulator of host-pathogen interactions in human viral infections. J Biomed Sci. 2016;23(1):74. doi:10.1186/s12929-016-0292-x.
60.
Mesri EA, Feitelson MA, Munger K. Human viral oncogenesis: a cancer hallmarks analysis. Cell Host Microbe. 2014;15(3):266–282. doi:10.1016/j.chom.2014.02.011.
61.
Zuo L, Yue W, Du S, et al. An update: Epstein-Barr virus and immune evasion via microRNA regulation. Virol Sin. 2017;32(3):175–187. doi:10.1007/s12250-017-3996-5.
62.
Dawidowicz M, Kula A, Mielcarska S, et al. miREIA – an immunoassay method in assessment of microRNA levels in tumor tissue-pilot study. The impact of miR-93-5p, miR-142-5p and IFNγ on PD-L1 level in colorectal cancer. Acta Biochim Pol. 2021;68(2):247–254. doi:10.18388/abp.2020_5533.
63.
Kappel A, Backes C, Huang Y, et al. MicroRNA in vitro diagnostics using immunoassay analyzers. Clin Chem. 2015;61(4):600–607. doi:10.1373/clinchem.2014.232165.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.