Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
RESEARCH PAPER
Evaluation of antioxidant activity of NG-R1 saponin against bacterial cells in induced oxidative stress – a preliminary study
1
Department of Pharmaceutical Microbiology and Microbiological Diagnostic, Medical University of Lodz, Łódź, Poland
Corresponding author
Anna Lichota
Department of Pharmaceutical Microbiology and Microbiological Diagnostic, Medical University of Lodz, Muszynskiego 1, 90-151, Lodz, Poland
Department of Pharmaceutical Microbiology and Microbiological Diagnostic, Medical University of Lodz, Muszynskiego 1, 90-151, Lodz, Poland
Ann Agric Environ Med. 2023;30(1):83-89
KEYWORDS
TOPICS
ABSTRACT
Introduction and objective:
Notoginsenoside R1 (NG-R1) is isolated from Panax notoginseng, a medicinal herb well-known for its long use in traditional Chinese medicine. NG-R1 is relatively under-studied in research on bacteria. The aim of the study was to investigate antioxidant properties of NG-R1 saponin of selected bacterial strains of intestinal microbiota that may be involved in the pathogenesis of thromboembolic diseases. Enterococcus faecalis and Escherichia coli were used in the study.
Material and methods:
The study determined the concentration of hydroperoxides, the level of lipid peroxidation, as well as carbonyl groups and free thiol groups. The research carried out in this way will allow determination of the influence of the above factors on bacteria living in intestinal microbiota.
Results:
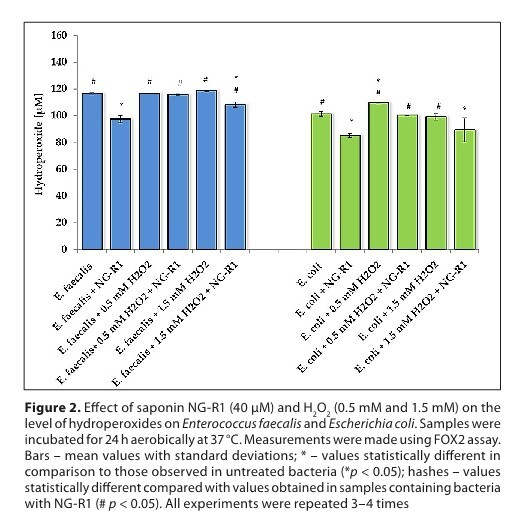
An evaluation of selected parameters of oxidative stress allowed to check whether the tested compound could reduce the pro-thrombotic activity of bacteria that were stimulated with H2O2. It was found that NG-R1 reduced hydroperoxide levels in both types of bacteria. In turn, lipid peroxidation initiated by H2O2 was suppressed by NG-R1. Hydrogen peroxide led to a strong increase in the level of carbonyl groups in Enterococcus faecalis and, to a lesser extent, in Escherichia coli. The addition of NG-R1 to the medium significantly reduced the level of carbonyls. Additionally, NG-R1 also induced a significant increase in the level of free thiol groups.
Conclusions:
Obtained results indicate that NG-R1 may have a protective effect on the intestinal microbiom through mechanisms involving changes in the redox state.
Notoginsenoside R1 (NG-R1) is isolated from Panax notoginseng, a medicinal herb well-known for its long use in traditional Chinese medicine. NG-R1 is relatively under-studied in research on bacteria. The aim of the study was to investigate antioxidant properties of NG-R1 saponin of selected bacterial strains of intestinal microbiota that may be involved in the pathogenesis of thromboembolic diseases. Enterococcus faecalis and Escherichia coli were used in the study.
Material and methods:
The study determined the concentration of hydroperoxides, the level of lipid peroxidation, as well as carbonyl groups and free thiol groups. The research carried out in this way will allow determination of the influence of the above factors on bacteria living in intestinal microbiota.
Results:
An evaluation of selected parameters of oxidative stress allowed to check whether the tested compound could reduce the pro-thrombotic activity of bacteria that were stimulated with H2O2. It was found that NG-R1 reduced hydroperoxide levels in both types of bacteria. In turn, lipid peroxidation initiated by H2O2 was suppressed by NG-R1. Hydrogen peroxide led to a strong increase in the level of carbonyl groups in Enterococcus faecalis and, to a lesser extent, in Escherichia coli. The addition of NG-R1 to the medium significantly reduced the level of carbonyls. Additionally, NG-R1 also induced a significant increase in the level of free thiol groups.
Conclusions:
Obtained results indicate that NG-R1 may have a protective effect on the intestinal microbiom through mechanisms involving changes in the redox state.
ACKNOWLEDGEMENTS
The authors would also like to thank Ms Dorota Wawrzyniak,
from the Foreign Language Centre of the Medical University
of Lodz, Poland for a language consultation.
REFERENCES (52)
1.
Jansen VL, Gerdes VE, Middeldorp S, et al. Gut microbiota and their metabolites in cardiovascular disease. Best Pract Res Clin Endocrinol Metab. 2021;35(3):101492. https://doi.org/10.1016/j.beem....
2.
Meng X, Sun G, Ye J, et al. Notoginsenoside R1-mediated neuro-protection involves estrogen receptor-dependent crosstalk between Akt and ERK1/2 pathways: A novel mechanism of Nrf2/ARE signaling activation. Free Radic Res. 2014;48(4):445–460. https://doi.org/10.3109/107157....
3.
Park SK, Hyun SH, In G, et al. The antioxidant activities of Korean Red Ginseng (Panax ginseng) and ginsenosides: A systemic review through in vivo and clinical trials. J Ginseng Res. 2021;45(1):41–47. https://doi.org/10.1016/j.jgr.....
4.
Chen XJ, Zhang XJ, Shui YM, et al. Anticancer Activities of Proto panaxadiol- and Protopanaxatriol-Type Ginsenosides and Their Metabolites. Evidence-based Complement. Altern Med. 2016;2016:5738694. https://doi.org/10.1155/2016/5....
5.
Liu H, Yang J, Yang W, et al. Focus on notoginsenoside R1 in metabolism and prevention against human diseases. Drug Des Devel Ther. 2020;14:551–565. https://doi.org/10.2147/DDDT.S....
6.
Tang Z, Yang C, He Z, et al. Notoginsenoside R1 alleviates spinal cord injury through the miR-301a/KLF7 axis to activate Wnt/ß-catenin pathway. Open Med. 2022;17(1):741–755. https://doi.org/10.1515/med-20....
7.
Dong Y, Yan X, Yang X, et al. Notoginsenoside R1 suppresses miR-301a via NF-?B pathway in lipopolysaccharide-treated ATDC5 cells. Exp Mol Pathol. 2020;112:104355. https://doi.org/10.1016/j.yexm....
8.
Fan C, Qiao Y, Tang M, et al. Notoginsenoside R1 attenuates high glucose-induced endothelial damage in rat retinal capillary endothelial cells by modulating the intracellular redox state. Drug Des Devel Ther. 2017;11:3343–3354. https://doi.org/10.2147/DDDT.S....
9.
Yu Y, Sun G, Luo Y, et al. Cardioprotective effects of Notoginsenoside R1 against ischemia/reperfusion injuries by regulating oxidative stress- and endoplasmic reticulum stress-related signaling pathways. Sci Rep. 2016;6:21730. https://doi.org/10.1038/srep21....
10.
Fang H, Yang S, Luo Y, et al. Notoginsenoside R1 inhibits vascular smooth muscle cell proliferation, migration and neointimal hyperplasia through PI3K/Akt signaling. Sci Rep. 2018;8(1):7595. https://doi.org/10.1038/s41598....
11.
Yang X, Xiong X, Wang H, et al. Protective effects of panax notoginseng saponins on cardiovascular diseases: A comprehensive overview of experimental studies. Evidence-based Complement. Altern Med. 2014;2014:204840. https://doi.org/10.1155/2014/2....
12.
Zhao H, Han Z, Li G, et al. Therapeutic potential and cellular mechanisms of Panax notoginseng on prevention of aging and cell senescence-associated diseases. Aging Dis. 2017;8(6):721–739. https://doi.org/10.14336/AD.20....
13.
Murphy K, O’donovan AN, Caplice NM, et al. Exploring the gut microbiota and cardiovascular disease. Metabolites. 2021;11(8):493. https://doi.org/10.3390/metabo....
14.
Kazemian N, Mahmoudi M, Halperin F, et al. Gut microbiota and cardiovascular disease: opportunities and challenges. Microbiome 2020;8(1):36. https://doi.org/10.1186/s40168....
15.
Formes H, Reinhardt C. The gut microbiota–a modulator of endothelial cell function and a contributing environmental factor to arterial thrombosis. Expert Rev Hematol. 2019;12(7):541–549. https://doi.org/10.1080/174740....
16.
Luz PL da, Haas EA, Favarato D. Intestinal Microbiota and Cardiovascular Diseases. Int J Cardiovasc Sci. 2020;33(5):462–471. https://doi.org/10.36660/ijcs.....
17.
Sun K, Wang C-SS, Guo J, et al. Protective effects of ginsenoside Rb1, ginsenoside Rg1, and notoginsenoside R1 on lipopolysaccharideinduced microcirculatory disturbance in rat mesentery. Life Sci. 2007;81(6):509–518. https://doi.org/10.1016/j.lfs.....
18.
Hasan RA, Koh AY, Zia A. The gut microbiome and thromboembolism. Thromb Res. 2020;189:77–87. https://doi.org/10.1016/j.thro....
19.
Suganya K, Son T, Kim K-W, et al. Impact of gut microbiota: How it could play roles beyond the digestive system on development of cardiovascular and renal diseases. Microb Pathog. 2021;152:104583. https://doi.org/10.1016/j.micp....
20.
Lichota A, Gwozdzinski K, Szewczyk EM. Microbial modulation of coagulation disorders in venous thromboembolism. J Inflamm Res. 2020;13:387–400. https://doi.org/10.2147/JIR.S2....
21.
Fejes AV, Best MG, van der Heijden WA, et al. Impact of Escherichia coli K12 and O18:K1 on human platelets: Differential effects on platelet activation, RNAs and proteins. Sci Rep. 2018;8(1):16145. https://doi.org/10.1038/s41598....
22.
Huycke MM. Enterococcus faecalis produces extracellular superoxide and hydrogen peroxide that damages colonic epithelial cell DNA. 2002;23(3):529–36. https://doi.org/doi.org/10.109....
23.
Van Tyne D, Martin MJ, Gilmore MS. Structure, function, and biology of the Enterococcus faecalis cytolysin. Toxins (Basel). 2013;5(5):895–911. https://doi.org/10.3390/toxins....
24.
Nogueira A, Peixoto F, Oliveira MM, et al. The Effects of Long-Term Chaetomellic Acid A Administration on Renal Function and Oxidative Stress in a Rat Model of Renal Mass Reduction. Biomed Res Int. 2017;2017:5125980. https://doi.org/10.1155/2017/5....
25.
Lopes D, Melo T, Santos N, et al. Evaluation of the interplay among the charge of porphyrinic photosensitizers, lipid oxidation and photoinactivation efficiency in Escherichia coli. J Photochem Photobiol B Biol. 2014;141:145–153. https://doi.org/10.1016/j.jpho....
26.
Álvarez MC, Caldiz C, Fantinelli JC, et al. Is cardiac hypertrophy in spontaneously hypertensive rats the cause or the consequence of oxidative stress? Hypertens Res. 2008;31(7):1465–1476. https://doi.org/10.1291/hypres....
27.
Czubak-Prowizor K, Trelinski J, Stelmach P, et al. Increased Oxidative Stress in Acute Myeloid Leukemia Patients after Red Blood Cell Transfusion, but Not Platelet Transfusion, Results Mainly from the Oxidative/Nitrative Protein Damage: An Exploratory Study J Clin Med. 2021;10(7):1349. https://doi.org/10.3390/jcm100....
28.
Stefek M, Trnkova Z, Krizanova L. 2,4-Dinitrophenylhydrazine carbonyl assay in metal-catalysed protein glycoxidation. Redox Rep. 1999;4(1–2):43–48. https://doi.org/10.1179/135100....
29.
Dalle-Donne I, Rossi R, Giustarini D, et al. Protein carbonyl groups as biomarkers of oxidative stress. Clin Chim Acta 2003;329(1–2):23–38. https://doi.org/ 10.1016/s0009-8981(03)00003-2.
30.
Semchyshyn H, Bagnyukova T, Storey K, et al. Hydrogen peroxide increases the activities of regulon enzymes and the levels of oxidized proteins and lipids in. Cell Biol Int. 2005;29(11):898–902. https://doi.org/10.1016/j.cell....
31.
Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82(1):70–77. https://doi.org/ 10.1016/0003-9861(59)90090-6.
32.
Gwozdzinski K, Pieniazek A, Brzeszczynska J, et al. Alterations in Red Blood Cells and Plasma Properties after Acute Single Bout of Exercise. Sci World J. 2013;2013:168376. https://doi.org/10.1155/2013/1....
33.
Girgin Ersoy Z, Barisci S, Dinc O. Mechanisms of the Escherichia coli and Enterococcus faecalis inactivation by ozone. LWT 2019;100:306–313. https://doi.org/10.1016/j.lwt.....
34.
Reinhardt C. The Gut Microbiota as an Influencing Factor of Arterial Thrombosis. Hamostaseologie 2019;39(2):173–179. https://doi.org/10.1055/s-0038....
35.
Haghikia A, van Mens TE, Pontarollo G, et al. Editorial: Impact of the gut microbiota on cardiovascular medicine. Front Med. 2022;9:939890. https://doi.org/10.3389/fmed.2....
36.
Masenga SK, Hamooya B, Hangoma J, et al. Recent advances in modulation of cardiovascular diseases by the gut microbiota. J Hum Hypertens. 2022;36(11):952–959. https://doi.org/10.1038/s41371....
37.
Fu J, Bonder MJ, Cenit MC, et al. The Gut Microbiome Contributes to a Substantial Proportion of the Variation in Blood Lipids. Circ Res. 2015;117(9):817–824. https://doi.org/10.1161/CIRCRE....
38.
Shandilya S, Kumar S, Kumar Jha N, et al. Interplay of gut microbiota and oxidative stress: Perspective on neurodegeneration and neuroprotection. J Adv Res. 2022;38: 223–244. https://doi.org/10.1016/j.jare....
39.
Lichota A, Szewczyk EM, Gwozdzinski K. Factors Affecting the Formation and Treatment of Thrombosis by Natural and Synthetic Compounds. Int J Mol Sci. 2020;21(21):7975. https://doi.org/10.3390/ijms21....
40.
Cha M-K, Hong S-K, Lee D-S, et al. Vibrio cholerae Thiol Peroxidase-Glutaredoxin Fusion Is a 2-Cys TSA/AhpC Subfamily Acting as a Lipid Hydroperoxide Reductase. J Biol Chem. 2004;279(12):11035–11041. https://doi.org/10.1074/jbc.M3....
41.
Alves E, Santos N, Melo T, et al. Photodynamic oxidation of Escherichia coli membrane phospholipids: New insights based on lipidomics. Rapid Commun. Mass Spectrom. 2013;27(23):2717–2728. https://doi.org/10.1002/rcm.67....
42.
Yan X, Budin-Verneuil A, Verneuil N, et al. Response of Enterococcus faecalis V583 to Low Hydrogen Peroxide Levels. Curr Microbiol. 2015;70(2):156–168. https://doi.org/10.1007/s00284....
43.
Rodríguez-Rojas A, Kim JJ, Johnston PR, et al. Non-lethal exposure to H2O2 boosts bacterial survival and evolvability against oxidative stress. PLOS Genet. 2020;16(3):e1008649. https://doi.org/10.1371/journa....
44.
Peters LP, Carvalho G, Martins PF, et al. Differential Responses of the Antioxidant System of Ametryn and Clomazone Tolerant Bacteria. PLoS One 2014;9(11):e112271. https://doi.org/10.1371/journa....
45.
Cong S, Xiang L, Yuan X, et al. Notoginsenoside R1 up-regulates microRNA-132 to protect human lung fibroblast MRC-5 cells from lipopolysaccharide-caused injury. Int Immunopharmacol. 2019;68:137–144. https://doi.org/10.1016/j.inti....
46.
Gui D, Wei L, Jian G, et al. Notoginsenoside R1 Ameliorates Podo cyte Adhesion Under Diabetic Condition Through ?3ß1 Integrin Upregulation in Vitro and in Vivo. Cell Physiol Biochem. 2014;34(6):1849–1862. https://doi.org/10.1159/000366....
47.
Zhang H-SS, Wang S-QQ. Notoginsenoside R1 inhibits TNF-?-induced fibronectin production in smooth muscle cells via the ROS/ERK pathway. Free Radic Biol Med. 2006;40(9):1664–1674. https://doi.org/10.1016/j.free....
48.
Xu H, Zhang X, Shi Y, et al. Notoginsenoside R1 relieves the myocardial infarction via activating the JAK2/STAT3 signaling pathway in vivo and in vitro. Bioengineered. 2022;13(3):5653–5662. https://doi.org/10.1080/216559....
49.
Zhang W, Xiao S, Ahn DU. Protein Oxidation: Basic Principles and Implications for Meat Quality. Crit. Rev. Food Sci Nutr. 2013;53(11):1191–1201. https://doi.org/10.1080/104083....
50.
Davies MJ. Protein oxidation and peroxidation. Biochem J. 2016;473(7):805–825. https://doi.org/10.1042/BJ2015....
51.
Luo D. Smith SW, Anderson BD, Kinetics and Mechanism of the Reaction of Cysteine and Hydrogen Peroxide in Aqueous Solution. J Pharm Sci. 2005;94(2):304–316. https://doi.org/10.1002/jps.20....
52.
Sjöberg B, Foley S, Cardey B, et al. Methionine oxidation by hydrogen peroxide in peptides and proteins: A theoretical and Raman spectroscopy study. J Photochem Photobiol B Biol. 2018;188:95–99. https://doi.org/10.1016/j.jpho....
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.