Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
REVIEW PAPER
Influence of pre- pro- and synbiotics on the proper functioning of the Gut-Brain Axis and cognition in exposure to stress – review of the latest scientific reports from in vivo studies
1
Institute of Rural Health, Lublin, Poland
Corresponding author
Ann Agric Environ Med. 2022;29(3):326-335
KEYWORDS
TOPICS
ABSTRACT
Introduction and objective:
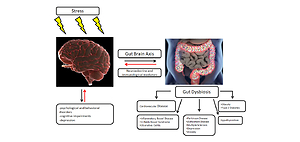
Every day, the human body is exposed to various stressors. Constant stress leads to a gradual weakening of the body, the disturbance of homeostasis and the emergence of somatic symptoms that require treatment. Due to chronic stress exposure, it seems important to support the body with natural supplementation, such as pre- pro- or synbiotics. The aim of the study was to review the latest reports from in vivo studies on the role of pre- pro- and synbiotics on the microbiota and the gut-brain axis (GBA) in stress exposure.
Review methods:
The article is an overview of literature reports from the past 5 years including in vivo studies examining the impact of pre- pro- and symbiotics on the gut microbiota and the GBA under stress conditions.
Abbreviated description of the state of knowledge:
Data from the World Health Organization (WHO) indicate that continuous exposure to stress contributes to up to 60% of chronic diseases. An important role in responding to stress is played by a properly functioning microbiome. Numerous studies concerning the pathology of the digestive system and disturbed microbiome show their relationship with nervous system disorders, thus confirming the importance of a proper bidirectional communication between these 2 systems.
Summary:
Daily exposure to stress may disrupt the microbiota and proper functioning of the GBA. The cortisol released in response to stress is an essential physiological response that helps with coping in threatening situations. However, its release triggers a cascade of subsequent biochemical reactions dangerous to health, especially if they are triggered too often, i.e. under chronic stress. The latest scientific reports from in vivo studies clearly show that proper supplementation and diet (used with caution) can be a potential add-on therapy in the treatment of neuropsychiatric disorders.
Every day, the human body is exposed to various stressors. Constant stress leads to a gradual weakening of the body, the disturbance of homeostasis and the emergence of somatic symptoms that require treatment. Due to chronic stress exposure, it seems important to support the body with natural supplementation, such as pre- pro- or synbiotics. The aim of the study was to review the latest reports from in vivo studies on the role of pre- pro- and synbiotics on the microbiota and the gut-brain axis (GBA) in stress exposure.
Review methods:
The article is an overview of literature reports from the past 5 years including in vivo studies examining the impact of pre- pro- and symbiotics on the gut microbiota and the GBA under stress conditions.
Abbreviated description of the state of knowledge:
Data from the World Health Organization (WHO) indicate that continuous exposure to stress contributes to up to 60% of chronic diseases. An important role in responding to stress is played by a properly functioning microbiome. Numerous studies concerning the pathology of the digestive system and disturbed microbiome show their relationship with nervous system disorders, thus confirming the importance of a proper bidirectional communication between these 2 systems.
Summary:
Daily exposure to stress may disrupt the microbiota and proper functioning of the GBA. The cortisol released in response to stress is an essential physiological response that helps with coping in threatening situations. However, its release triggers a cascade of subsequent biochemical reactions dangerous to health, especially if they are triggered too often, i.e. under chronic stress. The latest scientific reports from in vivo studies clearly show that proper supplementation and diet (used with caution) can be a potential add-on therapy in the treatment of neuropsychiatric disorders.
ACKNOWLEDGEMENTS
This research was funded by the National Science Center, Poland, grant UMO-2019/35/N/NZ7/00982.
REFERENCES (96)
1.
Antoniuk S, Bijata M, Ponimaskin E, Wlodarczyk J. Chronic unpredictable mild stress for modeling depression in rodents: Meta-analysis of model reliability. Neurosci Biobehav Rev. 2019;99:101–116. https://doi.org/10.1016/j.neub....
2.
Skonieczna-Żydecka K, Łoniewski I, Maciejewska D, Marlicz W. Mikrobiota jelitowa i składniki pokarmowe jako determinanty funkcji układu nerwowego. Aktualn Neurol. 2017;17:181–188.
3.
Herman JP, McKlveen JM, Ghosal S, et al. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr Physiol. 2016;6(2):603–621. https://doi.org/10.1002/cphy.c....
4.
Won E, Kim YK. Stress, the Autonomic Nervous System, and the Immune-kynurenine Pathway in the Etiology of Depression. Curr Neuropharmacol. 2016;14(7):665–73. https://doi.org/10.2174/157015....
5.
McEwen BS. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiological Reviews. 2007;87(3):873–904.
6.
Lupien SJ, Maheu F, Tu M, Fiocco A, Schramek TE. The effects of stress and stress hormones on human cognition: implications for the field of brain and cognition. Brain Cogn. 2007; 65:209–237.
7.
Payne JD, Jackson ED, Hoscheidt S, Ryan L, Jacobs WJ, Nadel L. Stress administered prior to encoding impairs neutral but enhances emotional long-term memories. Learn Mem. 2007;14:861– 868.
8.
Wolf OT. The influence of stress hormones on emotional memory: relevance for psychopathology. Acta Psychol (Amst). 2008;127:513–531.
9.
Kluen LM, Agorastos A, Wiedemann K, Schwabe L. Noradrenergic Stimulation Impairs Memory Generalization in Women. J Cogn Neurosci. 2017;29(7):1279–1291. https://doi.org/ 10.1162/jocn_a_01118.
10.
Gupta A, Saha S, Khanna S. Therapies to modulate gut microbiota: Past, present and future. World J Gastroenterol. 2020;26(8):777–788. https://doi.org/10.3748/wjg.v2....
11.
Rinninella E, Raoul P, Cintoni M, et al. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms. 2019;7(1):14. https://doi.org/10.3390/microo....
12.
Hasan N, Yang H. Factors affecting the composition of the gut microbiota, and its modulation. Peer J. 2019;7:7502. https://doi.org/10.7717/peerj.....
13.
Laterza L, Rizzatti G, Gaetani E, Chiusolo P, Gasbarrini A. The gut microbiota and immune system relationship in human graft-versus-host disease. Mediterr J Hematol Infect Dis. 2016;8:e2016025. https://doi.org/10.4084/mjhid.....
14.
Woźniak D, Cichy W, Przysławski J, Drzymała-Czyż S. The role of microbiota and enteroendocrine cells in maintaining homeostasis in the human digestive tract. Advances in Medical Sciences. 2021;66(2):284–292. https://doi.org/10.1016/j.advm....
15.
Kennedy PJ, Cryan JF, Dinan TG, Clarke G. Irritable bowel syndrome: a microbiome-gut-brain axis disorder? World J Gastroenterol. 2014;20:14105–14125.
16.
Karlsson F, Tremaroli V, Nielsen J, Bäckhed F. Assessing the human gut microbiota in metabolic diseases.Diabetes.2013;62:3341–3349.
17.
Bisgaard H, Li N, Bonnelykke K, Chawes BL, Skov T, Paludan-Müller G, Stokholm J, Smith B, Krogfelt KA. Reduced diversity of the intestinal microbiota during infancy is associated with increased risk of allergic disease at school age. J Allergy Clin Immunol.2011;128:646–652.
18.
Macfarlane S, Macfarlane GT. Regulation of short-chain fatty acid production. Proc Nutr Soc. 2003;62:67–72.
19.
Bourriaud C, Akoka S, Goupry S, Robins R, Cherbut C, Michel C. Butyrate production from lactate by human colonic microflora. Reprod Nutr Dev. 2002;42:55.
20.
Kaufman DW, Kelly JP, Curhan GC, Anderson TE, Dretler SP, Preminger GM, Cave DR. Oxalobacter formigenes may reduce the risk of calcium oxalate kidney stones. J Am Soc Nephrol. 2008;19:1197–1203. https://doi.org/10.1681/ASN.20....
21.
Jandhyala SM, Talukdar R, Subramanyam C, Vuyyuru H, Sasikala M, Nageshwar Reddy D. Role of the normal gut microbiota. World J Gastroenterol. 2015;21(29):8787–803. https://doi.org/10.3748/wjg.v2....
22.
Devillard E, McIntosh FM, Duncan SH, Wallace RJ. Metabolism of linoleic acid by human gut bacteria: different routes for biosynthesis of conjugated linoleic acid. J Bacteriol. 2007;189:2566–2570.
23.
Kim YS, Ho SB. Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr Gastroenterol Rep. 2010;12:319–330.
24.
Muniz LR, Knosp C, Yeretssian G. Intestinal antimicrobial peptides during homeostasis, infection, and disease. Front Immunol. 2012;3:310. https://doi.org/10.3389/fimmu.....
25.
Sekirov I, Shannon L, Russell L, Caetano M. Gut Microbiota in Health and Disease. Physiol Rev. 2010;1:859 –904. https://doi.org/10.1152/physre....
26.
Zheng D, Liwinski T. Interaction between microbiota and immunity in health and disease. Eran Elinav Cell Res. 2020;30(6):492–506. https://doi.org/10.1038/s41422....
27.
Hapfelmeier S, Lawson MA, Slack E, Kirundi JK, Stoel M, Heikenwalder M, Cahenzli J, Velykoredko Y, Balmer ML, Endt K, Geuking MB, et al. Reversible microbial colonization of germ-free mice reveals the dynamics of IgA immune responses. Science.2010;328:1705–1709.
28.
Ivanov II, FrutosRde L, Manel N, Yoshinaga K, Rifkin DB, Sartor RB, Finlay BB, Littman DR. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe.2008;4:337–349.
29.
Zhou L, Lopes JE, Chong MM, Ivanov II, Min R, Victora GD, Shen Y, Du J, Rubtsov YP, Rudensky AY, Ziegler SF, Littman DR. TGF-ß-induced Foxp3 inhibits TH17 cell differentiation by antagonizing ROR?t function. Nature.2008;453:236–240.
30.
Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immuno-modulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118.
31.
Collado MC, Rautava S, Aakko J, Isolauri E, Salminen S. Human gut colonization may be initiated in utero by distinct microbialcommunities in the placenta and amniotic fluid. Scientific Reports. 2016;6(1):23129. https://doi.org/10.1038/srep23....
32.
Dominguez-Bello MG, Costello EK, Contreras M, Magris M, Hidalgo G, Fierer N, Knight R. Delivery mode shapes the acquisition and structure of the initial microbiota across multiple body habitats in newborns. Proc Natl Acad Sci USA. 2010;107(26):11971–11975. https://doi.org/ 10.1073/pnas.1002601107.
33.
Aagaard K, Riehle K, Ma J, Segata N, Mistretta TA, Coarfa C, Raza S, Rosenbaum S, Van den Veyver I, Milosavljevic A, Gevers D, Huttenhower C, Petrosino J, Versalovic J. A metagenomic approach to characterization of the vaginal microbiome signature in pregnancy. PLoS One. 2012;7(6):e36466. https://doi.org/10.1371/journa....
34.
Madan JC, Hoen AG, Lundgren SN, et al. Association of Cesarean Delivery and Formula Supplementation With the Intestinal Microbiome of 6-Week-Old Infants. JAMA Pediatr. 2016;170(3):212–219. https://doi.org/10.1001/jamape....
35.
Plaza-Díaz J, Fontana L, Gil A. Human Milk Oligosaccharides and Immune System Development Nutrients. 2018;10(8):1038.
36.
Diaz Heijtz R. Fetal, neonatal, and infant microbiome: perturbations and subsequent effects on brain development and behavior. Semin Fetal Neonatal Med. 2016;21:410–417.
37.
Goyal MS, Venkatesh S, Milbrandt J, et al. Feeding the brain and nurturing the mind: linking nutrition and the gut microbiota to brain development. Proc Natl Acad Sci USA. 2015;112:14105–14112.
38.
Osadchiy V, Martin CR, Mayer EA. The Gut-Brain Axis and the Microbiome: Mechanisms and Clinical Implications. Clin Gastroenterol Hepatol. 2019;17(2):322–332. https://doi.org/ 10.1016/j.cgh.2018.10.002.
39.
Institute of Medicine. Dietary reference intakes: energy, carbohydrates, fiber, fat, fatty acids, cholesterol, protein and amino acids. National Academies Press. 2005. Washington, DC, USA.
40.
Klingensmith NJ, Coopersmith CM. The gut as the motor of multiple organ dysfunction in critical illness. Critical Care Clinics. 2016;32(2):203–212. https://doi.org/10.1016/j.ccc.....
41.
Jernberg C, Löfmark S, Edlund C, Jansson JK. Long-term ecological impacts of antibiotic administration on the human intestinal microbiota. ISME Journal. 2007;1(1):56–66. https://doi.org/ 10.1038/ismej.2007.3.
42.
Jakobsson HE, Jernberg C, Andersson AF, Sjölund-Karlsson M, Jansson JK, Engstrand L. Short-term antibiotic treatment has differing long-term impacts on the human throat and gut microbiome. Plos one. 2010;5(3):e9836. https://doi.org/10.1371/journa....
43.
Dethlefsen L, Relman DA. Incomplete recovery and individualized responses of the human distal gut microbiota to repeated antibiotic perturbation. Proc Natl Acad Sci USA. 2011;108 (1):4554–61. https://doi.org/10.1073/pnas.1....
44.
Huerta-Franco MR, Vargas-Luna M, Tienda P, Delgadillo-Holtfort I, Balleza-Ordaz M, Flores-Hernandez C. Effects of occupational stress on the gastrointestinal tract. World J Gastrointest Pathophysiol. 2013;4(4):108–118. https://doi.org/10.4291/wjgp.v....
45.
Pohl CS, Medland JE, Moeser AJ. Early-life stress origins of gastrointestinal disease: animal models, intestinal pathophysiology, and translational implications. Am J Physiol Gastrointest Liver Physiol. 2015; 309(12):927–941.
46.
Rosa V, Pallotta L, Cappelletti M, Severi C, Matarrese P. The Impact of Oxidative Stress in Human Pathology: Focus on Gastrointestinal Disorders. Antioxidants (Basel). 2021;10(2): 201. https://doi.org/10.3390/antiox....
47.
Konturek PC, Brzozowski T, Konturek SJ. Stress and the gut: pathophysiology, clinical consequences, diagnostic approach and treatment options. J Physiol Pharmacol. 2011;62(6): 591–599.
48.
Yahfoufi N, Matar C, Ismail N. Adolescence and Aging: Impact of Adolescence Inflammatory Stress and Microbiota Alterations on Brain Development, Aging, and Neurodegeneration. J Gerontol A Biol Sci Med Sci. 2020;75(7):1251–1257. https://doi.org/10.1093/gerona....
49.
Rudzki L, Frank M, Szulc A, et al. Od jelit do depresji – rola zaburzeń ciągłości bariery jelitowej i następcza aktywacja układu immunologicznego w zapalnej hipotezie depresji. Neuropsychiatria I Neuropsychologia. 2012;7(2): 76–84.
50.
Mohajeri MH, La Fata G, Steinert RE, Weber P. Relationship between the gut microbiome and brain function.Nutr. Rev. 2018;76(7):481–496.
51.
Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28:203–209.
52.
Adak A, Khan MR. An insight into gut microbiota and its functionalities. Cell Mol Life Sci. 2019;76(3):473–493. https://doi.org/10.1007/s00018....
53.
Badawy AA. Tryptophan availability for kynurenine pathway metabolism across the life span: control mechanisms and focus on aging, exercise, diet and nutritional supplements. Neuropharmacology. 2017;112:248–263.
54.
Mittal R, Debs LH, Patel AP, Nguyen D, Patel K, O’Connor G, Grati M, Mittal J, Yan D, Eshraghi AA, Deo SK, Daunert S, Liu XZ. Neurotransmitters: The Critical Modulators Regulating Gut–Brain Axis. J Cell Physiol. 2017;232:2359–2372. https://doi.org/10.1002/jcp.25....
55.
Silva YP, Bernardi A, Frozza RL. The Role of Short-Chain Fatty Acids From Gut Microbiota in Gut-Brain Communication. Front Endocrinol (Lausanne).2020;11:25. https://doi.org/ 10.3389/fendo.2020.00025.
56.
Bosi A, Banfi D, Bistoletti M, Giaroni C, Baj A. Tryptophan Metabolites Along the Microbiota-Gut-Brain Axis: An Interkingdom Communication System Influencing the Gut in Health and Disease. Int J Tryptophan Res. 2020;13:1178646920928984. https://doi.org/10.1177/117864....
57.
Sherwin E, Rea K, Dinan TG, Cryan JF. A gut (microbiome) feeling about the brain. CurrOpinGastroenterol. 2016;32(2):96–102.
58.
Tomova A, Husarova V, Lakatosova S, Bakos J, Vlkova B, Babinska K, Ostatnikova D. Gastrointestinal microbiota in children with autism in Slovakia. Physiol Behav. 2015;138:179–87. https://doi.org/10.1016/j.phys....
59.
Akbari E, Asemi Z, Daneshvar Kakhaki R, Bahmani F, Kouchaki E, Tamtaji OR, Hamidi GA, Salami M. Effect of Probiotic Supplementation on Cognitive Function and Metabolic Status in Alzheimer’s Disease: A Randomized, Double-Blind and Controlled Trial. Front Aging Neurosci. 2016;8:256. https://doi.org/10.3389/fnagi.....
60.
Fröhlich EE, Farzi A, Mayerhofer R, Reichmann F, Jačan A, Wagner B, Zinser E, Bordag N, Magnes C, Fröhlich E, Kashofer K, Gorkiewicz G, Holzer P. Cognitive impairment by antibiotic-induced gut dysbiosis: Analysis of gut microbiota-brain communication. Brain Behav Immun. 2016;56:140–55. https://doi.org/10.1016/j.bbi.....
61.
Nimgampalle M, Kuna Y. Anti-Alzheimer Properties of Probiotic, Lactobacillus plantarum MTCC 1325 in Alzheimer’s Disease induced Albino Rats. J Clin Diagn Res. 2017;11(8):1–5. https://doi.org/10.7860/JCDR/2....
62.
Athari Nik Azm S, Djazayeri A, Safa M, Azami K, Ahmadvand B, Sabbaghziarani F, Sharifzadeh M, Vafa M. Lactobacilli and bifidobacteria ameliorate memory and learning deficits and oxidative stress in ß-amyloid (1–42) injected rats. Appl Physiol Nutr Metab. 2018;43(7):718–726. https://doi.org/10.1139/apnm-2....
63.
Zeng M, Inohara N, Nunez G. Mechanisms of inflammation driven bacterial dysbiosis in the gut. Mucosal Immunol. 2017;10:18.
64.
Tetel MJ, de Vries GJ, Melcangi RC, Panzica G, O’Mahony SM. Steroids, stress and the gut microbiome-brain axis. J Neuroendocrinol.2018;30(2):10. https://doi.org/10.1111/jne.12....
65.
Molina-Torres G, Rodriguez-Arrastia M, Roman P, Sanchez-Labraca N, Cardona D. Stress and the gut microbiota-brain axis. Behav Pharmacol. 2019;30:187–200. https://doi.org/ 10.1097/FBP.0000000000000478.
66.
Sanders ME, Heimbach JT, Pot B, Tancredi DJ, Lenoir-Wijnkoop I, Lähteenmäki-Uutela A, Gueimonde M, Banares S. Health claims substantiation for probiotic and prebiotic products. Gut Microbes. 2011;2(3):127–33. https://doi.org/10.4161/gmic.2....
67.
European Food Safety Authority (EFSA) Scientific Opinion on the update of the list of QPS-recommended biological agents intentionally added to food or feed as notified to EFSA J. 2017;15:1–177.
68.
Markowiak P, Śliżewska K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients. 2017;9(9):1021. https://doi.org/10.3390/nu9091....
69.
Kim SK, Guevarra RB, Kim YT, Kwon J, Kim H, Cho JH, Kim HB, Lee JH. Role of Probiotics in Human Gut Microbiome-Associated Diseases. J Microbiol Biotechnol. 2019;29(9):1335–1340. https://doi.org/10.4014/jmb.19....
70.
Long-Smith C, O’Riordan KJ, Clarke G, Stanton C, Dinan TG, Cryan JF. Microbiota-Gut-Brain Axis: New Therapeutic Opportunities. Annu Rev Pharmacol Toxicol. 2020;60:477–502. https://doi.org/10.1146/annure....
71.
Gibson GR, Scott KP, Rastall RA, Tuohy KM, Hotchkiss A, Dubert-Ferrandon A, Gareau M, Murphy EF, Saulnier D, Loh G, et al. Dietary prebiotics: Current status and new definition. Food Sci Technol Bull Funct Foods. 2010;7:1–19. https://doi.org/10.1616/1476-2....
72.
Varzakas T, Kandylis P, Dimitrellou D, Salamoura C, Zakynthinos G, Proestos C. Preparation and Processing of Religious and Cultural Foods. Elsevier.2018;1:67–129.
73.
Louis P, Flint HJ, Michel C. Microbiota of the Human Body. Springer; 2016. p.119–142.
74.
Tzounis X, Rodriguez-Mateos A, Vulevic J, Gibson GR, Kwik-Uribe C, Spencer JP. Prebiotic evaluation of cocoa-derived flavanols in healthy humans by using a randomized, controlled, double-blind, crossover intervention study. Am J Clin Nutr. 2011;93:62–72. https://doi.org/10.3945/ajcn.1....
75.
Wang S, Xiao Y, Tian F, Zhao J, Zhang H, et al. Rational use of prebiotics for gut microbiota alterations: Specific bacterial phylotypes and related mechanisms. J Functional Foods. 2020;66. https://doi.org//10.1016/j.jff....
76.
Swanson KS, Gibson GR, Hutkins R, Reimer RA, Reid G, Verbeke K, Scott KP, Holscher HD, Azad MB, Delzenne NM, et al. The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of synbiotics. Nat Rev Gastroenterol Hepatol. 2020;17:687–701. https://doi.org/10.1038/s41575....
77.
Manigandan T, Mangaiyarkarasi SP, Hemaltha R, Hemaltha VT, Murali NP. Probiotics, prebiotics and synbiotics—A review. Biomed Pharmacol J.2012;5:295–304. https://doi.org/10.13005/bpj/3....
78.
Burokas A, Arboleya S, Moloney RD, Peterson VL, Murphy K, Clarke G, Stanton C, Dinan TG, Cryan JF. Targeting the Microbiota-Gut-Brain Axis: Prebiotics Have Anxiolytic and Antidepressant-like Effects and Reverse the Impact of Chronic Stress in Mice. Biol Psychiatry. 2017;82(7):472–487. https://doi.org/10.1016/j.biop....
79.
Li H, Wang P, Huang L, Li P, Zhang D. Effects of regulating gut microbiota on the serotonin metabolism in the chronic unpredictable mild stress rat model. Neurogastroenterol Motil. 2019;31(10):13677. https://doi.org/10.1111/nmo.13....
80.
Mika A, Day HE, Martinez A, Rumian NL, Greenwood BN, Chichlowski M, Berg BM, Fleshner M. Early life diets with prebiotics and bioactive milk fractions attenuate the impact of stress on learned helplessness behaviours and alter gene expression within neural circuits important for stress resistance. Eur J Neurosci. 2017;45(3):342–357. https://doi.org/10.1111/ejn.13....
81.
Qiu ZK, Liu CH, Gao ZW, et al. The inulin-type oligosaccharides extract from morinda officinalis, a traditional Chinese herb, ameliorated behavioral deficits in an animal model of post-traumatic stress disorder. Metab Brain Dis. 2016;31:1143–1149. https://doi.org//10.1007/s1101....
82.
Chi L, Khan I, Lin Z, Zhang J, Lee MYS, Leong W, Hsiao WLW, Zheng Y. Fructo-oligosaccharides from Morinda officinalis remodeled gut microbiota and alleviated depression features in a stress rat model. Phytomedicine. 2020;67:153157. https://doi.org/10.1016/j.phym....
83.
Neufeld KA, O’Mahony SM, Hoban AE, Waworuntu RV, Berg BM, Dinan TG, Cryan JF. Neurobehavioural effects of Lactobacillus rhamnosus GG alone and in combination with prebiotics polydextrose and galactooligosaccharide in male rats exposed to early-life stress. Nutr Neurosci. 2019;22(6):425–434. https://doi.org/10.1080/102841....
84.
Barrera-Bugueno C, Realini O, Escobar-Luna J, Sotomayor-Zárate R, Gotteland M, Julio-Pieper M, Bravo JA. Anxiogenic effects of a Lactobacillus, inulin and the synbiotic on healthy juvenile rats. Neuroscience. 2017;359:18–29. https://doi.org/10.1016/j.neur....
85.
Gu F, Wu Y, Liu Y, Dou M, Jiang Y, Liang H. Lactobacillus casei improves depression-like behavior in chronic unpredictable mild stress-induced rats by the BDNF-TrkB signal pathway and the intestinal microbiota. Food Funct. 2020;11(7):6148–6157. https://doi.org/10.1039/d0fo00....
86.
Li N, Wang Q, Wang Y, Sun A, Lin Y, Jin Y, Li X. Oral Probiotics Ameliorate the Behavioral Deficits Induced by Chronic Mild Stress in Mice via the Gut Microbiota-Inflammation Axis. Front Behav Neurosci. 2018;12:266. https://doi.org/10.3389/fnbeh.....
87.
Sun J, Wang F, Hu X, et al. Clostridium butyricum Attenuates Chronic Unpredictable Mild Stress-Induced Depressive-Like Behavior in Mice via the Gut-Brain Axis. J Agric Food Chem. 2018;66(31):8415–8421. https://doi.org/10.1021/acs.ja....
88.
Tian P, Wang G, Zhao J, Zhang H, Chen W. Bifidobacterium with the role of 5-hydroxytryptophan synthesis regulation alleviates the symptom of depression and related microbiota dysbiosis. J Nutr Biochem. 2019;66:43–51. https://doi.org/10.1016/j.jnut....
89.
Sun Y, Geng W, Pan Y, Wang J, Xiao P, Wang Y. Supplementation with Lactobacillus kefiranofaciens ZW3 from Tibetan Kefir improves depression-like behavior in stressed mice by modulating the gut microbiota. Food Funct. 2019;10(2):925–937. https://doi.org/10.1039/c8fo02....
90.
Hao Z, Wang W, Guo R, Liu H. Faecalibacterium prausnitzii (ATCC 27766) has preventive and therapeutic effects on chronic unpredictable mild stress-induced depression-like and anxiety-like behavior in rats. Psychoneuroendocrinology. 2019;104:132–142. https://doi.org/10.1016/j.psyn....
91.
Guo Y, Xie JP, Deng K, et al. Prophylactic Effects of Bifidobacterium adolescentis on Anxiety and Depression-Like Phenotypes After Chronic Stress: A Role of the Gut Microbiota-Inflammation Axis. Front Behav Neurosci. 2019;13:126. https://doi.org/10.3389/fnbeh.....
92.
Tian P, O’Riordan KJ, Lee YK, Wang G, Zhao J, Zhang H, Cryan JF, Chen W. Towards a psychobiotic therapy for depression: Bifidobacterium breve CCFM1025 reverses chronic stress-induced depressive symptoms and gut microbial abnormalities in mice. Neurobiol Stress. 2020;12:100216. https://doi.org/10.1016/j.ynst....
93.
Kochalska K, Oakden W, Słowik T, Chudzik A, Pankowska A, Łazorczyk A, Kozioł P, Andres-Mach M, Pietura R, Rola R, Stanisz GJ, Orzylowska A. Dietary supplementation with Lactobacillus rhamnosus JB-1 restores brain neurochemical balance and mitigates the progression of mood disorder in a rat model of chronic unpredictable mild stress. Nutr Res. 2020;82:44–57. https://doi.org/10.1016/j.nutr....
94.
Chudzik A, Słowik T, Kochalska K, Pankowska A, Łazorczyk A, Andres-Mach M, Rola R, Stanisz GJ, Orzyłowska A. Continuous Ingestion of Lacticaseibacillus rhamnosus JB-1 during Chronic Stress Ensures Neurometabolic and Behavioural Stability in Rats. Int J Mol Sci. 2022;23(9):5173. https://doi.org/10.3390/ijms23....
95.
Ding Y, Bu F, Chen T, Shi G, Yuan X, Feng Z, Duan Z, Wang R, Zhang S, Wang Q, Zhou J, Chen Y. A next-generation probiotic: Akkermansia muciniphila ameliorates chronic stress-induced depressive-like behavior in mice by regulating gut microbiota and metabolites. Appl Microbiol Biotechnol. 2021;105(21–22):8411–8426. https://doi.org/10.1007/s00253....
96.
Gao K, Farzi A, Ke X, Yu Y, Chen C, Chen S, Yu T, Wang H, Li Y. Oral administration of Lactococcus lactis WHH2078 alleviates depressive and anxiety symptoms in mice with induced chronic stress. Food Funct. 2022;13(2):957–969. https://doi.org/10.1039/d1fo03....
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.