Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
CASE REPORT
First report of subcutaneous Taenia crassiceps cysticercosis in a dog in Poland
1
Department of Infectious and Invasive Diseases and Veterinary Administration, Institute of Veterinary Medicine, Faculty of Biological and Veterinary Sciences, Nicolaus Copernicus University, Toruń, Poland
2
Department of Parasitology and Molecular Biology, Animallab Veterinary Laboratory, Poland
3
Canine and Feline Nutrition Specialist, Pupil Veterinary Clinic, Karolina Ściubisz, DVM, Gliwice, Poland
4
Veterinary Clinic, AniCura, Gliwice, Poland
5
Department of Internal Diseases with Clinic for Horses, Dogs and Cats, Faculty of Veterinary Medicine, University of Environmental and Life Sciences, Wrocław, Poland
6
Department of Small Animal Diseases with Clinic, Institute of Veterinary Medicine, Warsaw University of Life Sciences-SGGW, Warsaw, Poland
Corresponding author
Dawid Jańczak
Department of Infectious and Invasive Diseases and Veterinary Administration, Institute of Veterinary Medicine, Faculty of Biological and Veterinary Sciences, Nicolaus Copernicus University, Lwowska 1, 87-100, Toruń, Poland
Department of Infectious and Invasive Diseases and Veterinary Administration, Institute of Veterinary Medicine, Faculty of Biological and Veterinary Sciences, Nicolaus Copernicus University, Lwowska 1, 87-100, Toruń, Poland
KEYWORDS
TOPICS
ABSTRACT
A 3-year-old male German Shepherd with Addison’s disease, hematological abnormalities, and chronic glucocorticoid therapy
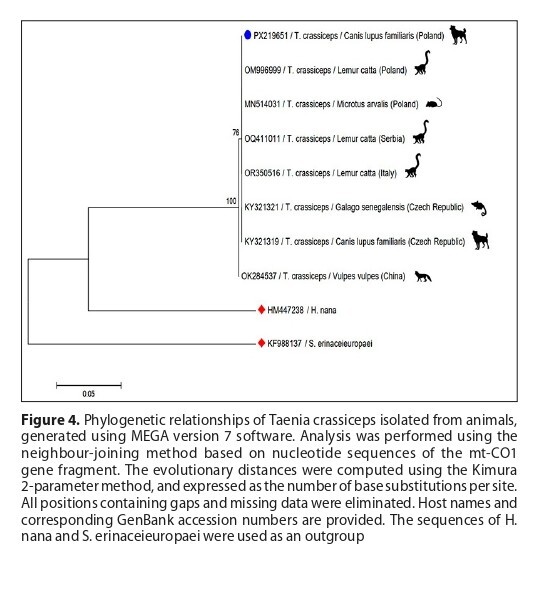
developed subcutaneous nodules containing abundant cestode larvae. Microscopy, PCR, and mtCO1 sequencing confirmed
Taenia crassiceps metacestodes (GenBank accession no. PX219651). This unusual case illustrates a domestic dog as an aberrant
intermediate host with both subcutaneous and peritoneal cysticercosis, likely favored by long-term immunosuppression. The
findings align with previous reports in immunocompromised hosts and emphasize clinical awareness of atypical parasitic
infections. Cytology and molecular assays proved valuable for diagnosis. Given the zoonotic potential of T. crassiceps and
its broad host range, early recognition in immunocompromised animals is critical for intervention, case management, and
prevention. A One Health approach integrating veterinary and medical perspectives is essential to reduce its impact as a
parasitic threat.
REFERENCES (27)
1.
Zhang Y, Abdu A, Wu T, et al. Taenia crassiceps cysticercosis in a wild muskrat and a domestic dog in the northeastern United States. Pathogens. 2023;12(2):204. https://doi.org/10.3390/pathog....
2.
Bajer A, Alsarraf M, Dwużnik D, et al. Rodents as intermediate hosts of cestode parasites of mammalian carnivores and birds of prey in Poland, with the first data on the life-cycle of Mesocestoides melesi. Parasites Vectors. 2020;13(1):95. https://doi.org/10.1186/s13071....
3.
Bowman DD. Georgis’ Parasitology for Veterinarians. 11th ed. Philadelphia, PA: Saunders; 2020:414–415.
4.
Bagnato E, Lauthier JJ, Brook F, et al. Natural life cycle and molecular characterization of Taenia talicei Dollfus, 1960 (Cestoda: Taeniidae) from northwestern Patagonia, Argentina. Int J Parasitol Parasites Wildl. 2024;26:101035. https://doi.org/10.1016/j.ijpp....
5.
Freeman RS. Studies on the biology of Taenia crassiceps (Zeder, 1800) Rudolph, 1810 (Cestoda). Can J Zool. 1962;40:969–990. https://doi.org/10.1139/z62-08....
6.
Ganzorig S, Gardner SL. Eucestoda. In: Gardner SL, Gardner SA, editors. Concepts in Animal Parasitology. Lincoln, NE: Zea Books; 2024:251–261.
7.
Citterio CV, Obber F, Trevisiol K, et al. Echinococcus multilocularis and other cestodes in red foxes (Vulpes vulpes) of northeast Italy, 2012–2018. Parasites Vectors. 2021;14:29. https://doi.org/10.1186/s13071....
8.
Karamon J, Sroka J, Dąbrowska J, et al. First report of Echinococcus multilocularis in cats in Poland: a monitoring study in cats and dogs from a rural area and animal shelter in a highly endemic region. Parasites Vectors. 2019;12(1):313. https://doi.org/10.1186/s13071....
9.
Schneider A, Moré G, Pewsner M, et al. Cestodes in Eurasian wolves (Canis lupus lupus) and domestic dogs (Canis lupus familiaris) in Switzerland. Int J Parasitol Parasites Wildl. 2024;26:101027. https://doi.org/10.1016/j.ijpp....
10.
Federer K, Armua-Fernandez MT, Gori F, et al. Detection of taeniid (Taenia spp., Echinococcus spp.) eggs contaminating vegetables and fruits sold in European markets and the risk for metacestode infections in captive primates. Int J Parasitol Parasites Wildl. 2016;5(3):249–253. https://doi.org/10.1016/j.ijpp....
11.
Deplazes P, Eichenberger RM, Grimm F. Wildlife-transmitted Taenia and Versteria cysticercosis and coenurosis in humans and other primates. Int J Parasitol Parasites Wildl. 2019;9:342–358. https://doi.org/10.1016/j.ijpp....
12.
Tappe D, Berkholz J, Mahlke U, et al. Molecular Identification of Zoonotic Tissue-Invasive Tapeworm Larvae Other than Taenia solium in Suspected Human Cysticercosis Cases. J Clin Microbiol. 2016;54(1):172–174. https://doi.org/10.1128/JCM.02....
13.
Reuter A, Wennemuth J. Computed tomographic appearance of canine hepatic alveolar echinococcosis. Vet Radiol Ultrasound. 2024;65(2):138–144. https://doi.org/10.1111/vru.13....
14.
Hoberg EP, Ebinger W, Render JA. Fatal cysticercosis by Taenia crassiceps (Cyclophyllidea: Taeniidae) in a presumed immunocompromised canine host. J Parasitol. 1999;85(6):1174–1178. https://doi.org/10.2307/328567....
15.
Nolte A, Strube C, Raue K, et al. Subkutane Taenia crassiceps-Zystizerkose bei einem Hund mit Cushing-Syndrom [Subcutaneous Taenia crassiceps cysticercosis in a dog with Cushing’s syndrome]. Tierarztl Prax Ausg K Kleintiere Heimtiere. 2016;44(1):53–58. https://doi.org/10.15654/TPK-1...
16.
Samorek-Pieróg M, Karamon J, Brzana A, et al. Molecular confirmation of Taenia crassiceps cysticercosis in a captive ring-tailed lemur (Lemur catta) in Poland. Pathogens. 2022;11:835. https://doi.org/10.3390/pathog....
17.
Bowles J, Blair D, McManus DP. Genetic variants within the genus Echinococcus identified by mitochondrial DNA sequencing. Mol Biochem Parasitol. 1992;54(2):165–173. https://doi.org/10.1016/0166-6....
18.
Cuccato M, Rubiola S, Rossi L, et al. Massive infection by Cysticercus longicollis in a captive Lemur catta from Italy: a case report. Front Vet Sci. 2023;10:1288451. https://doi.org/10.3389/fvets.....
19.
Simin S, Vračar V, Kozoderović G, et al. Subcutaneous Taenia crassiceps cysticercosis in a ring-tailed lemur (Lemur catta) in a Serbian zoo. Acta Parasitol. 2023;68(2):468–472. https://doi.org/10.1007/s11686....
20.
Grbavac L, Šikić A, Kostešić P, et al. Comprehensive diagnosis, treatment, and outcome of Taenia crassiceps cysticercosis in a ring-tailed lemur (Lemur catta) from a Croatian zoo: no longer unusual? Pathogens. 2024;13(4):283. https://doi.org/10.3390/pathog....
21.
Alić A, Hodžić A, Škapur V, et al. Fatal pulmonary cysticercosis caused by Cysticercus longicollis in a captive ring-tailed lemur (Lemur catta). Vet Parasitol. 2017;241:1–4. https://doi.org/10.1016/j.vetp....
22.
Floß N, Dolff S, Junker A, et al. Cerebral Taenia crassiceps larvae infection in a 71-year-old immunocompetent male. Infection. 2023;51(1):277–281. https://doi.org/10.1007/s15010....
23.
Murphy C, Kursh L, Nolan T, et al. Subcutaneous Taenia crassiceps cysticercosis mass excision from an 11-year-old mixed-breed dog. J Am Anim Hosp Assoc. 2021;57(5):1–5. https://doi.org/10.5326/JAAHA-....
24.
Hofmannová L, Mikeš L, Jedličková L, et al. Unusual cases of Taenia crassiceps cysticercosis in naturally infected animals in the Czech Republic. Vet Med (Praha). 2018;63:73–80. https://doi.org/10.17221/58/20....
25.
Romano MC, Valdéz RA, Cartas AL, et al. Steroid hormone production by parasites: the case of Taenia crassiceps and Taenia solium cysticerci. J Steroid Biochem Mol Biol. 2003;85(2–5):221–225. https://doi.org/10.1016/S0960-....
26.
Schoenle LA, Moore IT, Dudek AM, et al. Exogenous glucocorticoids amplify the costs of infection by reducing resistance and tolerance, but effects are mitigated by co-infection. Proc Biol Sci. 2019;286(1900):20182913. https://doi.org/10.1098/rspb.2....
27.
da Silva Santana RC, Prudente TP, de Sousa Guerra CH, et al. Albendazole–ivermectin combination decreases inflammation in experimental neurocysticercosis. Exp Parasitol. 2023;251:108568. https://doi.org/10.1016/j.expp....
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.