Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
RESEARCH PAPER
Elucidating environmental reservoir of antimicrobial resistance – a phenotypic characterization of gut microbiota from aquatic coleoptera in a low-anthropogenic impact zone
1
Vocational School of Health Services at Atatürk University, Erzurum, Türkiye
2
Hınıs Vocational Collage, Atatürk University, Erzurum, Türkiye
3
Department of Biology, Science Faculty, Kyrgyz-Turkish Manas University, Bishkek, Kyrgyzstan
4
Department of Biology, Science Faculty, Ataturk University, Erzurum, Türkiye
These authors had equal contribution to this work
Corresponding author
Mehmet Bektaş
Hınıs Vocational Collage, Hınıs Vocational Collage, Ataturk University, Hınıs, 25600, Erzurum, Turkey
Hınıs Vocational Collage, Hınıs Vocational Collage, Ataturk University, Hınıs, 25600, Erzurum, Turkey
Ann Agric Environ Med. 2025;32(4):504-510
KEYWORDS
TOPICS
- Biological agents posing occupational risk in agriculture, forestry, food industry and wood industry and diseases caused by these agents (zoonoses, allergic and immunotoxic diseases)
- State of the health of rural communities depending on various factors: social factors, accessibility of medical care, etc.
ABSTRACT
Introduction and objective:
This study investigated the antibiotic resistance of bacterial isolates obtained from the gut microbiota of certain insects (Coleoptera: Hydrophilidae and Helophoridae), which were collected from aquatic areas in Erzurum Province, Türkiye. This area is characterised by a low level of human impact, thereby providing a unique opportunity to investigate the baseline microbial diversity and ecological roles within relatively pristine aquatic environments.
Material and methods:
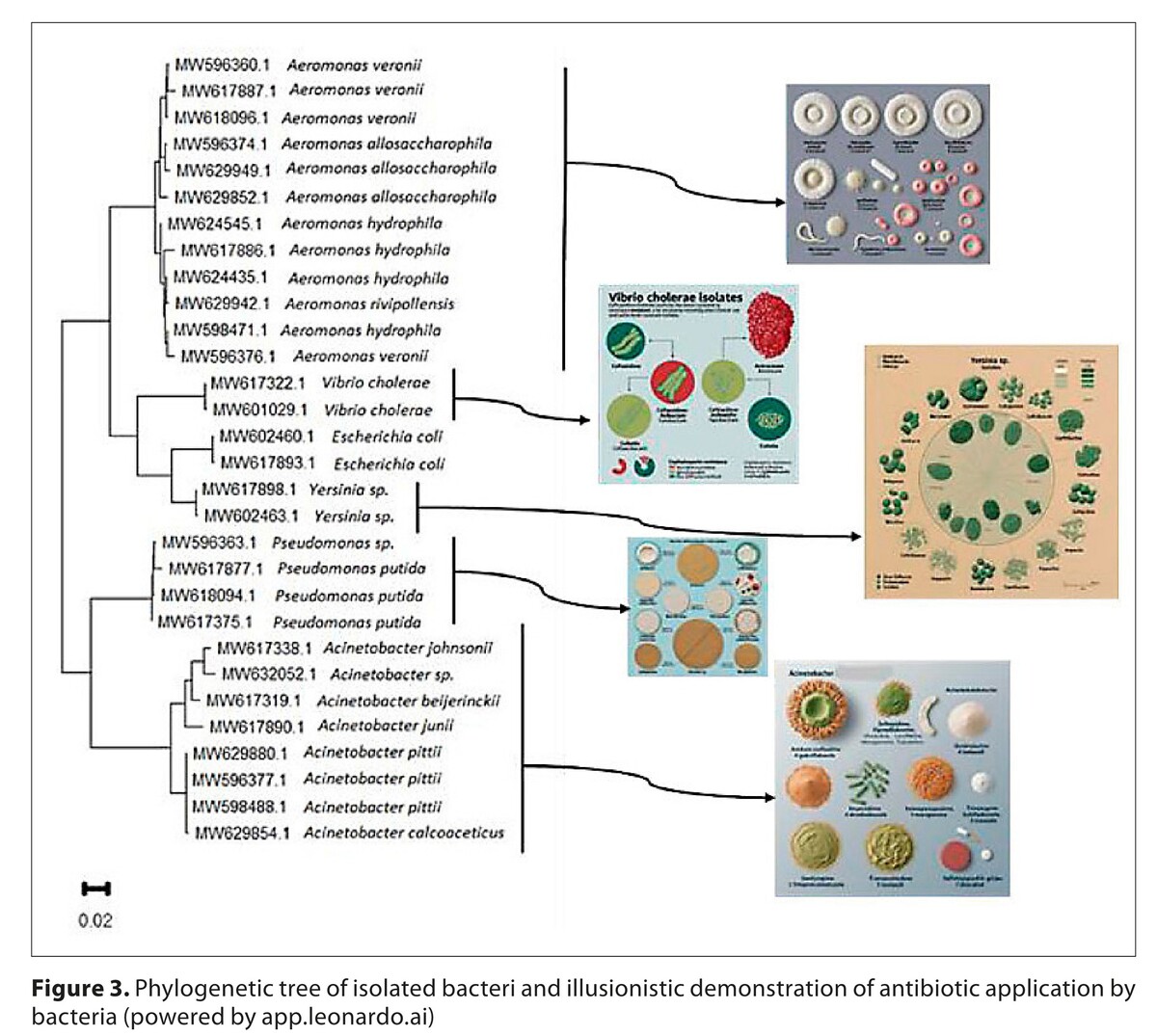
The antimicrobial susceptibility of the isolates was assessed using disc diffusion and minimum inhibitory concentration (MIC) methods. The analysis encompassed 30 Gram-negative bacteria belonging to the genera Aeromonas, Acinetobacter, Vibrio, Pseudomonas, Escherichia and Yersinia.
Results:
The results indicated that the most resistant bacteria were Aeromonas, Pseudomonas and Acinetobacter, while enteric bacteria demonstrated greater sensitivity. It is noteworthy that nitrofurantoin, a commonly used antibiotic for treating urinary tract infections, exhibited the highest level of resistance among the antibiotics tested by disc diffusion, followed by cephalosporins and penicillins.
Conclusions:
The MIC testing with DKGM and NF kits demonstrated high resistance to cephalosporins, sulfonamides, polymyxins and monobactams. Furthermore, two multidrug-resistant (MDR) isolates exhibited resistance to at least two antibiotic classes. These findings underscore the necessity for expanded antimicrobial resistance surveillance beyond clinical settings, extending into environmental samples, and contributing to ongoing research on resistance mechanisms.
This study investigated the antibiotic resistance of bacterial isolates obtained from the gut microbiota of certain insects (Coleoptera: Hydrophilidae and Helophoridae), which were collected from aquatic areas in Erzurum Province, Türkiye. This area is characterised by a low level of human impact, thereby providing a unique opportunity to investigate the baseline microbial diversity and ecological roles within relatively pristine aquatic environments.
Material and methods:
The antimicrobial susceptibility of the isolates was assessed using disc diffusion and minimum inhibitory concentration (MIC) methods. The analysis encompassed 30 Gram-negative bacteria belonging to the genera Aeromonas, Acinetobacter, Vibrio, Pseudomonas, Escherichia and Yersinia.
Results:
The results indicated that the most resistant bacteria were Aeromonas, Pseudomonas and Acinetobacter, while enteric bacteria demonstrated greater sensitivity. It is noteworthy that nitrofurantoin, a commonly used antibiotic for treating urinary tract infections, exhibited the highest level of resistance among the antibiotics tested by disc diffusion, followed by cephalosporins and penicillins.
Conclusions:
The MIC testing with DKGM and NF kits demonstrated high resistance to cephalosporins, sulfonamides, polymyxins and monobactams. Furthermore, two multidrug-resistant (MDR) isolates exhibited resistance to at least two antibiotic classes. These findings underscore the necessity for expanded antimicrobial resistance surveillance beyond clinical settings, extending into environmental samples, and contributing to ongoing research on resistance mechanisms.
FUNDING
The study was supported financially by Atatürk University
Scientific Research Unit (Project No: TSA-2022–10866).
REFERENCES (25)
1.
Hughes AC, Tougeron DA, Martin F, et al. Smaller human populations are neither a necessary nor sufficient condition for biodiversity conservation. Bio Cons. 2023;277:109841. https://doi.org/10.1016/j.bioc....
2.
Elechi JOG, Ikechukwu UN, Cornelius SA. Global food system transformation for resilience. Food Systems Resilie. IntechOpen. 2022. https://doi.org/10.5772/intech....
3.
Gałęcki R, Zielonka Ł, Zasępa M, et al. Potential Utilization of Edible Insects as an Alternative Source of Protein in Animal Diets in Poland. Front Sustain Food Syst. 2021;5:675796. https://doi.org/10.3389/fsufs.....
4.
Stull VJ. Impacts of insect consumption on human health. J Ins Foo Fee. 2021;7:695–713. https://www.wageningenacademic....
5.
Stull VJ, Finer E, Bergmans RS, et al. Impact of edible cricket consumption on gut microbiota in healthy adults, a double-blind, randomized crossover trial. Sci Rep. 2018;8:10762. https://www.nature.com/article....
6.
Domínguez-Santos R, Pérez-Cobas AE, Cuti P, et al. Interkingdom gut microbiome and resistome of the cockroach Blattella germanica. Msystems. 2021;6:1110–28. https://journals.asm.org/doi/f....
7.
Serwecińska L. Antimicrobials and antibiotic-resistant bacteria: a risk to the environment and to public health. Water. 2020;12(3313):1–17. https://doi.org/10.1016/j.chem....
8.
Zhang Y, Cai T, Wan H. Mobile Resistance Elements: Symbionts That Modify Insect Host Resistance. J Agric Food Chem. 2025;73: 3842−3853. https://doi.org/10.1021/acs.ja....
9.
Lakbar I, De Waele JJ, Tabah A, et al. Antimicrobial de-escalation in the ICU: from recommendations to level of evidence. Adva in Ther. 2020;37:3083–3096. https://doi.org/10.1007/s12325....
10.
Gajic I, Tomic N, Lukovic B, et al. A comprehensive overview of antibacterial agents for combating Multidrug-Resistant bacteria: the current landscape, development, future opportunities, and challenges. Antibiotics. 2025;14(3–221):1–40. https://doi.org/10.3390/antibi....
11.
Gwenzi W, Chaukura N, Muisa-Zikali N, et al. Insects, rodents and pets as reservoirs, vectors and sentinels of antimicrobial resistance. Antibio. 2021;10:68. https://doi.org/10.3390/antibi....
12.
Rawat N, Sabu B, Jamwal R, et al. Understanding the role of insects in the acquisition and transmission of antibiotic resistance. Sci Tot Env. 2023;858:159805. https://doi.org/10.1016/j.scit....
13.
Siddiqui JA, Khan MM, Bamisile BS, et al. Role of insect gut microbiota in pesticide degradation: a review. Front Micro. 2022;13:870462. https://doi.org/10.3389/fmicb.....
14.
Salam MA, Al-Amin MY, Salam MT, et al. Antimicrobial Resistance: A Growing Serious Threat for Global Public Health. Healthcare. 2023;11:1946. https://doi.org/10.3390/health....
15.
Garofalo C, Milanović V, Cardinali F, et al. Current knowledge on the microbiota of edible insects intended for human consumption: A state-of-the-art review. Foo Res Int. 2019;125:108527. https://doi.org/10.1016/j.food....
16.
Turchi B, Mancini S, Pedonese F, et al. Antibiotic Resistance in Enterococci and Enterobacteriaceae from Laboratory-Reared Fresh Mealworm Larvae (Tenebrio molitor L.) and Their Frass. Pathogens. 2024;13(6):456. https://doi.org/10.3390/pathog....
17.
Crippen TL, Sullivan JP, Anderson RC. Bacterial proximity effects on the transfer of antibiotic resistance genes within the alimentary tract of yellow mealworm larvae. J Eco Entomo. 2024;117:417–426. https://doi.org/10.1093/jee/to....
18.
Breijyeh Z, Jubeh B, Karaman R. Resistance of gram-negative bacteria to current antibacterial agents and approaches to resolve it. Molecules. 2020;25:1340. https://doi.org/10.3390/molecu....
19.
Bektas M, Orhan F, Baris O. Isolation of biological control agents and biotechnological bacteria from aquatic insect gut microbiota (Coleoptera: Helophoridae, Hydrophilidae). Bio Bullet. 2022;49:596–608. https://doi.org/10.1134/S10623....
20.
Sroka J, Karamon J, Wójcik-Fatla A, et al. Toxoplasma gondii infection in selected species of free-living animals in Poland. Ann Agric Env Med. 2019;26(4):656–660. https://doi.org/10.26444/aaem/....
21.
Bisetegn FS, Azene H, Ahmed KS, et al. Extended spectrum and metalo beta lactamase producing gram negative bacterial pathogens from cockroaches collected at hospital, Southern Ethiopia. Ant Res Inf Con. 2024;13:87. https://doi.org/10.1186/s13756....
22.
Szewczyk A, Zagaja M, Bryda J, et al. Topinambur-new possibilities for use in a supplementation diet. Ann Agric Env Med. 2019;26(1):24–28. https://doi.org/10.26444/aaem/....
23.
Patil A, Singh N, Patwekar M, et al. AI-driven insights into the microbiota: Figuring out the mysterious world of the gut. Intel Pharm. 2025;3(1):46–52. https://doi.org/10.1016/j.ipha....
24.
Kiskó G, Bajramović B, Elzhraa F, et al. The Invisible Threat of Antibiotic Resistance in Food. Antibiotics. 2025;14(3):250. https://doi.org/10.3390/antibi....
25.
Denk-Lobnig M, Wood KB. Antibiotic resistance in bacterial communities. Curr Opin Microbiol. 2023;74:102306. https://doi.org/10.1016/j.mib.....
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.