Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
REVIEW PAPER
The impact of xenoestrogens on effectiveness of treatment for hormone-dependent breast cancer – current state of knowledge and perspectives for research
1
Department of Toxicology, Faculty of Pharmacy, Wroclaw Medical University, Poland
Ann Agric Environ Med. 2020;27(4):526-534
KEYWORDS
TOPICS
ABSTRACT
Introduction:
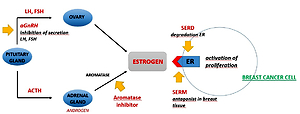
Breast cancer is the most common cancer occurring in women and causing the highest number of deaths among them. The role of xenoestrogens has been the subject of many studies in the pathogenesis of breast cancer. Less is known about the impact of xenoestrogens on the effectiveness of hormone therapy used to treat breast cancer, and thus possible drug-xenostrogen interactions.
Objective:
The aim of this review is to summarize the current state of knowledge and present perspectives for further research on the impact of xenoestrogens on the effectiveness of drugs used in the treatment of hormone-dependent breast cancer.
Current state of knowledge:
Phytoestrogens, in particular flavonoid genistein, are the best studied group of xenoestrogens in terms of interaction with drugs used in the treatment of breast cancer, due to their frequent use, including their use in alleviating the adverse effects of hormone therapy. Analyzing the current state of knowledge, it seems that phytoestrogens intake should be avoided during conventional anti-cancer treatment. Of the other xenoestrogens, bisphenol A (BPA) is one of the best-tested compounds for interactions with drugs used to treat breast cancer. It has been shown that bisphenol A could reduced therapeutic effect of active tamoxifen metabolite and cytostatics used in breast cancer treatment.
Conclusions:
Confirmation in clinical trials of the results obtained in vitro and in vivo tests, would enable the creation of specific recommendations for patients undergoing breast cancer treatment, especially hormone therapy. An area requiring further research is the analysis of the effects of xenoestrogens other than phytoestrogens, e.g. metalloestrogens, on the effects of drugs used in the treatment of breast cancer.
Breast cancer is the most common cancer occurring in women and causing the highest number of deaths among them. The role of xenoestrogens has been the subject of many studies in the pathogenesis of breast cancer. Less is known about the impact of xenoestrogens on the effectiveness of hormone therapy used to treat breast cancer, and thus possible drug-xenostrogen interactions.
Objective:
The aim of this review is to summarize the current state of knowledge and present perspectives for further research on the impact of xenoestrogens on the effectiveness of drugs used in the treatment of hormone-dependent breast cancer.
Current state of knowledge:
Phytoestrogens, in particular flavonoid genistein, are the best studied group of xenoestrogens in terms of interaction with drugs used in the treatment of breast cancer, due to their frequent use, including their use in alleviating the adverse effects of hormone therapy. Analyzing the current state of knowledge, it seems that phytoestrogens intake should be avoided during conventional anti-cancer treatment. Of the other xenoestrogens, bisphenol A (BPA) is one of the best-tested compounds for interactions with drugs used to treat breast cancer. It has been shown that bisphenol A could reduced therapeutic effect of active tamoxifen metabolite and cytostatics used in breast cancer treatment.
Conclusions:
Confirmation in clinical trials of the results obtained in vitro and in vivo tests, would enable the creation of specific recommendations for patients undergoing breast cancer treatment, especially hormone therapy. An area requiring further research is the analysis of the effects of xenoestrogens other than phytoestrogens, e.g. metalloestrogens, on the effects of drugs used in the treatment of breast cancer.
ACKNOWLEDGEMENTS
This research was financially supported by the Ministry of
Health subwention according to number of STM.D150.20.048.
from the IT Simple system of Wroclaw Medical University.
Boszkiewicz K, Sawicka E, Piwowar A. The impact of xenoestrogens on the effectiveness of treatment for hormone-dependent breast cancer
– current state of knowledge and perspectives for research. Ann Agric Environ Med. 2020; 27(4): 526–534. doi: 10.26444/aaem/124165
REFERENCES (63)
1.
Breast cancer. World Health Organization. Available on the Internet: https://www.who.int/cancer/pre... (access: 2020.03.02).
2.
Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin. 2019; 69: 7–34. https://doi.org/10.3322/caac.2....
3.
Krajowy Rejestr Nowotworów. Available on the Internet: http://onkologia.org.pl/ (access: 2020.03.02).
4.
Sowa M, Smuczyński W, Tarkowski M, Wójcik K, Kochański B. Analysis of the selected risk factors for breast cancer – literature review. J Educ Health Sport. 2015; 5: 245–250. https://dx.doi.org/10.5281/zen....
5.
Jouybari L, Saei Ghare Naz M, Sanagoo A, Kiani F, Sayehmiri F, Sayehmiri K, et al. Toxic elements as biomarkers for breast cancer: a meta-analysis study. Cancer Manag Res. 2018; 10: 69–79. https://doi.org/10.2147/CMAR.S....
6.
Kolak A, Kamińska M, Sygit K, Budny A, Kukiełka-Budny B, Burdan F. Primary and secondary preventionof breast cancer. Ann Agric Environ Med. 2017; 24(4): 549–553. https://doi.org/10.26444/aaem/....
7.
Paszko A, Krzyżak M, Charkiewicz AE, Ziembicka D, Żendzian-Piotrowska M, Szpak AS, Florek-Łuszczki M, Maślach D. Inequalities in breast cancer incidence and stage distribution between urban and rural female population in Świętokrzyskie Province, Poland. Ann Agric Environ Med. 2019; 26(1): 159–164. https://doi.org/10.26444/aaem/....
8.
Chang Y, Singh K. Long-term exposure to estrogen enhances chemotherapeutic efficacy potentially through epigenetic mechanism in human breast cancer cells. PLoS One. 2017; 12: e0174227. https://doi.org/10.1371/journa....
9.
Russo J, Russo I. The role of estrogen in the initation of breast cancer. J Steroid Biochem Mol Biol. 2006; 102: 89–96. https://doi.org/10.1016/j.jsbm....
10.
Dall G, Britt K. Estrogen effects on the mammary gland in early and late life and breast cancer risk. Front Oncol. 2017; 7: 110–117. https://doi.org/10.3389/fonc.2....
11.
Paruthiyil S, Parmar H, Kerekatte V, Cunha G, Firestone G, Leitman D. Estrogen receptor ß inhibits human breast cancer cell proliferation and tumour formation by causing a G2 cell cycle arrest. Cancer Res. 2004; 64: 423–428. https://doi.org/10.1158/0008-5....
12.
Świtalska M, Strządała L. Niegenomowe działanie estrogenów. Post Hig Med Dosw. 2007; 61: 541–547.
13.
Waks AG, Winer EP. Breast cancer treatment. A review. JAMA. 2019; 321(3): 288–300. https://doi.org/10.1001/jama.2....
14.
Jassem J, Krzakowski M, Bobek-Billewicz B, Duchnowska R, Jeziorski A, Olszewski W, et al. Breast cancer. Oncol Clin Pract. 2018; 14(4): 171–215. https://doi.org/10.5603/OCP.20....
15.
Glück S. Changing the gold standard in adjuvant therapy for breast cancer: from tamoxifen to aromatase inhibition. Biomed Pharmacother. 2005; 59 Suppl 2: 321–322. https://doi.org/10.1016/S0753-....
16.
Martinkovich S, Shah D, Planey SL, Arnott JA. Selective estrogen receptor modulators: tissue specificity and clinical utility. Clin Interv Aging. 2014; 9: 1437–1452. https://doi.org/10.2147/CIA.S6....
17.
Dębska-Szmich S, Zięba A, Potemski P. Fulvestrant in hormonal treatment of breast cancer. Oncol Clin Pract. 2017; 13: 14–23. https://doi.org/10.5603/OCP.20....
18.
Ortmann O, Weiss JM, Dietrich K. Gonadotrophin-releasing hormone (GnRH) and GnRH agonists: mechanisms of action. Reprod Biomed Online. 2002; 5 Suppl 1: 1–7. https://doi.org/10.1016/s1472-....
19.
Balic M, Thomssen C, Würstlein R, Gnant M, Harbeck N. St. Gallen/Vienna 2019: a brief of summary of the consensus discussion on the optimal primary breast cancer treatment. Breast Care. 2019; 14: 103–110. https://doi.org/10.1159/000499....
20.
Amaral Mendes JJ. The endocrine disrupters: a major medical challenge. Food Chem Toxicol. 2002; 40(6): 781–788. https://doi.org/10.1016/s0278-....
21.
Woźniak M, Murias M. Ksenoestrogeny: substancje zakłócające funkcjonowanie układu hormonalnego. Ginekol Pol. 2008; 9: 785–790.
22.
Langauer-Lewowicka H, Pawlas K. Endocrine disrupting chemicals – probability of adverse environmental effect. (Związki endokrynnie czynne – prawdopodobieństwo niepożądanego działania środo wiskowego). Med Środow. 2015; 18(1): 7–11.
23.
Taskinen H, Lindbohm ML, Sallmen M. Occupational exposure to chemicals and reproductive health. In: Gupta RC, eds. Reproductive and Developmental Toxicity. Elsevier Inc., London, Burlington, MA, San Diego, CA, 2011. p. 949–955.
24.
Hiatt RA, Brody JG. Environmental determinants of breast cancer. Ann Rev Public Health. 2018; 39: 113–133. https://doi.org/10.1146/annure....
25.
Terry MB, Michels KB, Brody JG. Envirinmental exposures during windows of susceptibility for breast cancer: a framework for prevention research. Breast Cancer Res. 2019; 21(1): 96. https://doi.org/10.1186/s13058....
26.
Mlynarcikova A, Macho L, Ficova M. Bisfenol A alone and in combination with estradiol modulates cell cycle and apoptosis-related proteins and genes in MCF-7 cells. Endocr Regul. 2013; 47(4): 189–99. https://doi.org/10.4149/endo_2....
27.
Fernandez S, Huang Y, Snider K, Zhou Y, Pogash T, Russo J. Expression and DNA methylation changes in human breast epithelial cells after bisphenol A (BPA) esposure. Int J Oncol. 2012; 41: 369–377. https://doi.org/10.3892/ijo.20....
28.
Kim J, Choi H, Lee H, Lee G, Hwang K, Choi K. Effects of bisphenol compounds on the growth and epithelial mesenchymal transition of MCF-7 CV human breast cancer cells. J Biomed Res. 2017; 31: 358–369. https://doi.org/10.7555/JBR.31....
29.
Wang Z, Liu H, Liu S. Low-dose bisphenol A exposure: a seemingly instigating carcinogenic effect on breast cancer. Adv Sci. 2016; 4(2): 1600248. https://doi.org/10.1002/advs.2....
30.
Chen F, Chien M, Chern I. Impact of low concentrations of phthalates on the effects of 17ß-estradiol in MCF-7 breast cancer cells. Taiwan J Obstet Gynecol. 2016; 55: 826–834. https://doi.org/10.1016/j.tjog....
31.
Wróbel A, Gregoraszczuk E. Effects of single and repeated in vitro exposure of three forms of parabens, methyl-, butyl- and propylparabens on the proliferation and estradiol secrection in MCF-7 and MCF-10A cells. Pharmacol Rep. 2013; 65: 484–493. https://doi.org/10.1016/s1734-....
32.
Luevano J, Damodaran C. A review of molecular events of cadmium-induced carcinogenesis. J Environ Pathol Toxiol Oncol. 2014; 33: 183–194. https://doi.org/10.1615/jenvir....
33.
Siewit C, Gengler B, Vegas E, Puckett R, Louie M. Cadmium promotes breast cancer cell proliferation by potentiating the interaction between ERalpha and c-Jun. Mol Endocrinol. 2010; 24: 981–992. https://doi.org/10.1210/me.200....
34.
Choe SY, Kim SJ, Kim HG, Lee JH, Choi Y, Lee H, et al. Evaluation of estrogenicity of major heavy metals. Sci Total Environ. 2003; 312: 15–21. https://doi.org/10.1016/S0048-....
35.
Sondergaard TE, Hansen FT, Purup S, Nielsen AK, Bonefeld-Jorgensen EC, Giese H, et al. Fusarin C acts like an estrogenic agonist and stimulates breast cancer cells in vitro. Toxicol Lett. 2011; 205: 116–121. https://doi.org/10.1016/j.toxl....
36.
Khosrokhavar R, Rahimifard N, Shoeibi S, Hamedani MP, Hosseini MJ. Effects of zearalenone and ?-zearalenol in comparison with raloxifene on T47D cells. Toxicol Mech Methods. 2009; 19: 246–250. https://doi.org/10.1080/153765....
37.
Duffy C, Perez K, Partridge A. Implications of phytoestrogen intake for breast cancer. CA Cancer J Clin. 2007; 57: 260–277. https://doi.org/10.3322/CA.57.....
38.
Lecomte S, Demay F, Ferriere F, Pakdel F. Phytochemicals targeting estrogen receptors: beneficial rather than adverse effects? Int J Mol Sci. 2017; 18(7). https://doi.org/10.3390/ijms18....
39.
van Duursen MBM, Smeets EJW, Rijk JCW, Nijmeijer SM, van den Berg M. Phytoestrogens in menopausal supplements induce ER-dependent cell proliferaton and overcome breast cancer treatment in an in vitro breast cancer model. Toxicol App Pharmacol. 2013; 269(2): 132–140. https://doi.org/10.1016/j.taap....
40.
Kennedy J, Wang CC, Wu CH. Patient disclosure about herb and supplement use among adults in the US. Evid Based Complement Alternat Med. 2007; 5: 451–456. https://doi.org/10.1093/ecam/n....
41.
Kowalczyk A, Bodalska A, Boszkiewicz K, Karłowicz-Bodalska K. Use of the herbal OTC products and dietary supplements by patients receiving chemotherapy: survey-based study. Indian J Pharm Educ Res. 2017; 51(4): 675–678. https://doi.org/10.5530/ijper.....
42.
Albini A, Rosano C, Angelini G, Amaro A, Esposito AI, Maramotti S, et al. Exogenous hormonal regulation in breast cancer cells by phytoestrogens and endocrine disruptors. Curr Med Chem. 2014; 21: 1129–1145. https://doi.org/10.2174/092986....
43.
Jones JL, Daley BJ, Enderson BL, Zhou JR, Karlstad MD. Genistein inhibits tamoxifen effects on cell proliferation and cell cycle arrest in T47D breast cancer cells. Am Surg. 2002; 68(6): 575–7. https://www.ncbi.nlm.nih.gov/p....
44.
Ju YH, Doerge DR, Allred KF, Allred CD, Helferich WG. Dietary genistein negates the inhibitory effect of tamoxifen on growth of estrogen-dependent human breast cancer (MCF-7) cells implanted in athymic mice. Cancer Res. 2002; 62(9): 2474–7. https://www.ncbi.nlm.nih.gov/p....
45.
Du M, Yang X, Hartman JA, Cooke PS, Doerge DR, Ju YH, et al. Low-dose dietary genistein negates the therapeutic effect of tamoxifen in anthymic nude mice. Carcinogenesis. 2012; 33(4): 895–901. https://doi.org/10.1093/carcin....
46.
Liu B, Edgerton S, Yang X, Kim A, Ordonez-Ercan D, Mason T, et al. Low-dose dietary phytoestrogen abrogates tamoxifen-associated mammary tumour prevention. Cancer Res. 2005; 65(3): 879–86. https://www.ncbi.nlm.nih.gov/p....
47.
Seo HS, DeNardo DG, Jacquot Y, Laios I, Salazar Vidal Doris, Rojas Zambrana C, et al. Stimulatory effect of genistein and apigenin on the growth of breast cancer cells correlates with their ability to activate ER alpha. Breast Cancer Res Treat. 2006; 99(2): 121–134. https://doi.org/10.1007/s10549....
48.
Constantinou AI, White BEP, Tonetti D, Yang Y, Liang W, Li W, et al. The soy isoflavone didzein improves the capacity of tamoxifen to prevent mammary tumours. Eur J Cancer. 2005; 41(4): 647–654. https://doi.org/10.1016/j.ejca....
49.
Kim YG, Park YH, Yang EY, Park WS, Park KS. Inhibition of tamoxifen’s therapeutic effects by emodin in estrogen receptor-positive breast cancer cell lines. Ann Surg Treat Res. 2019; 97(5): 230–238. https://doi.org/10.4174/astr.2....
50.
Ju YH, Doerge DR, Woodling KA, Hartman JA, Kwak J, Helferich WG. Dietary genistein negates the inhibitory effect of letrozole on the growth of aromatase-expressing estrogen-dependent human breast cancer cells (MCF-7Ca) in vivo. Carcinogenesis. 2008; 29(11): 2162–8. https://doi.org/10.1093/carcin....
51.
Nißlein T, Freudenstein J. Coadministration of the aromatase inhibitor formestane and an isopropanolic extract of black cohosh in a rat model of chemically induced mammary carcinoma. Planta Med. 2007; 73(4): 318–22. https://doi.org/10.1055/s-2007....
52.
Warth B, Raffeiner P, Granados A, Huan T, Fang M, Forsberg EM, et al. Metabolomics reveals that dietary xenoestrogens alter cellular metabolism induced by palbociclib/letrozole combination cancer therapy. Cell Chem Biol. 2018; 25(3): 291–300. https://doi.org/10.1016/j.chem....
53.
Gallo D, Mantuano E, Fabrizi M, Ferlini C, Mozzetti S, De Stefano I, et al. Effects of a phytoestrogen-containing soy extract on the growth-inhibitory activity of ICI 182 780 in an experimental model of estrogen-dependent breast cancer. Endocr Relat Cancer. 2007; 14(2): 317–324. https://doi.org/10.1677/ERC-06....
54.
Dees C, Foster JS, Ahamed S, Wimalasena J. Dietary estrogens stimulate human breast cells to enter the cell cycle. Environ Health Perspect. 1997; 105 Suppl 3: 633–6. April 1997. https://doi.org/10.1289/ehp.97....
55.
Aniogo EC, Plackal Adimuriyil George B, Abrahamse H. The role of photodynamic therapy on multidrug resistant breast cancer. Cancer Cell Int. 2019; 19: 91. https://doi.org/10.1186/s12935....
56.
Wang J, Seebacher N, Shi H, Kan Q, Duan Z. Novel strategies to prevent the development of multidrug resistance (MDR) in cancer. Oncotarget. 2017; 8(48): 84559–84571. doi: 10.18632/oncotarget.19187.
57.
Kim B, Fatayer H, Hanby AM, Horgan K, Perry SL. Neoadjuvant chemotherapy induces expression levels of breast cancer resistance protein that predict disease-free survival in breast cancer. PLoS One. 2013; 8; e62766. https://doi.org/10.1371/journa....
58.
Rigalli JP, Tocchetti GN, Arana MR, Villanueva SSM, Catania VA, Theile D, et al. The phytoestrogen genistein enhances multidrug resistance in breast cancer cell lines by translational regulation of ABC transporters. Cancer Lett. 2016; 376(1): 165–72. https://doi.org/10.1016/j.canl....
59.
Gao H, Yang BJ, Li N, Feng LM, Shi XY, Zhao WH, et al. Bisfenol A and hormone-associated cancers: current progress and perspectives. Medicine. 2015; 94(1): e211. https://doi.org/10.1097/MD.000....
60.
Goodson III AH, Luciani MG, Sayeed SA, Jaffee IM, Moore II DH, Dairkee SH. Activation of the mTOR pathway by low levels of xenoestrogens in breast epithelial cells from high-risk women. Carcinogenesis. 2011; 32(11): 1724–33. https://doi.org/10.1093/carcin....
61.
Dairkee SH, Luciani-Torres MG, Moore II DH, Goodson III AH. Bisphenol-A-induced inactivation of the p53 axis underlying deregulation of proliferation kinetics, and cell death in non-malignant human breast epithelial cells. Carcinogenesis. 2013; 13(3): 703–712. https://doi.org/10.1093/carcin....
62.
LaPensee EW, Tuttle TR, Fox SR, Ben-Jonathan N. Bisphenol A at low nanomolar doses confers chemoresistance in estrogen receptor-?-positive and -negative breast cancer cells. Environ Health Perspect. 2009; 117(2): 175–80. https://doi.org/10.1289/ehp.11....
63.
Osuna MAL, Nichols C, Perry C, Runke S, Krutilina, Seagroves TN, et al. Methylparaben stimulates tumour initiating cells in ER+ breast cancer models. J Appl Toxicol. 2017; 3(4): 417–425. https://doi.org/10.1002/jat.33....
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.