Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
REVIEW PAPER
The importance of gluten exclusion in the management of Hashimoto’s thyroiditis
1
University of Life Sciences, Warsaw, Poland
2
University of Agriculture, Kraków, Poland
Corresponding author
Małgorzata Ewa Drywień
Warsaw University of Life Sciences, Nowoursynowska 159 c, 02-776, Warsaw, Poland
Warsaw University of Life Sciences, Nowoursynowska 159 c, 02-776, Warsaw, Poland
Ann Agric Environ Med. 2021;28(4):558-568
KEYWORDS
TOPICS
ABSTRACT
Introduction and objective:
There is a growing interest in a gluten-free diet (GFD) in the management of various autoimmune diseases, including Hashimoto’s thyroiditis (HT). Even medical professionals claim that gluten elimination may improve a patient’s treatment. Some studies suggest a relationship between gluten intake and HT development or progression. The aim of the study was to analyze and describe available knowledge regarding the effect of gluten or a gluten-free diet on thyroid autoimmunity in HT with or without celiac disease.
Brief description of the state of knowledge:
Potentially applicable records were obtained through review and analysis of the PUBMED (MEDLINE) and Google Scholar database by using the following phrases: ‘hypothyroidism gluten’, ‘Hashimoto gluten’ and ‘thyroiditis gluten’. If a record focused on the subject by title and abstrakt, the full paper was screened. Authors’ scientific achievements and references of eligible records were screened for possibly omitted studies. The review was focused only on human studies.
Discussion:
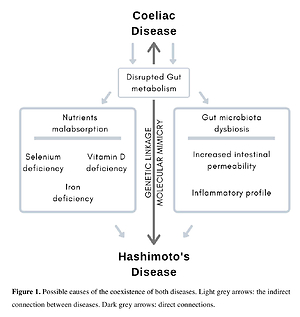
Gluten exclusion might increase the risk of HT development because of the potential nutritional deficiencies related to the low quality of gluten-free products. Gluten intake from crops grown on selenium-depleted soil increases the risk of HT development. Only a few studies suggest that GFD would be beneficial for HT patients, even without the coexistence of CD. The strongest connection between gluten intake and thyroid destruction seems to be based on a mechanism of molecular mimicry between gut and thyroid tissue transglutaminase.
Conclusions:
Studies conducted so far do not support the claim that HT patients should eliminate gluten from their diet. In view of the limited number of studies, with major limitations and ambiguous results, a gluten-free diet is not recommended.
There is a growing interest in a gluten-free diet (GFD) in the management of various autoimmune diseases, including Hashimoto’s thyroiditis (HT). Even medical professionals claim that gluten elimination may improve a patient’s treatment. Some studies suggest a relationship between gluten intake and HT development or progression. The aim of the study was to analyze and describe available knowledge regarding the effect of gluten or a gluten-free diet on thyroid autoimmunity in HT with or without celiac disease.
Brief description of the state of knowledge:
Potentially applicable records were obtained through review and analysis of the PUBMED (MEDLINE) and Google Scholar database by using the following phrases: ‘hypothyroidism gluten’, ‘Hashimoto gluten’ and ‘thyroiditis gluten’. If a record focused on the subject by title and abstrakt, the full paper was screened. Authors’ scientific achievements and references of eligible records were screened for possibly omitted studies. The review was focused only on human studies.
Discussion:
Gluten exclusion might increase the risk of HT development because of the potential nutritional deficiencies related to the low quality of gluten-free products. Gluten intake from crops grown on selenium-depleted soil increases the risk of HT development. Only a few studies suggest that GFD would be beneficial for HT patients, even without the coexistence of CD. The strongest connection between gluten intake and thyroid destruction seems to be based on a mechanism of molecular mimicry between gut and thyroid tissue transglutaminase.
Conclusions:
Studies conducted so far do not support the claim that HT patients should eliminate gluten from their diet. In view of the limited number of studies, with major limitations and ambiguous results, a gluten-free diet is not recommended.
REFERENCES (114)
1.
Szostak-Węgierek D, Bednarczuk T, Respondek W, Traczyk I, Cukrowska B, Ostrowska L, et al. The validity of using a gluten-free diet in Hashimoto’s disease: the position of the Expert Group of the Medical Dietetics Section of the Polish Society of Parenteral Nutrition and Enteral Metabolism (POLSPEN). Postępy Żywienia Klinicznego. 2018; 47: 33–47. [in Polish].
2.
Janatuinen EK, Kemppainen TA, Julkunen RJ, Kosma VM, Mäki M, Heikkinen M, Uusitupa MI. No harm from five year ingestion of oats in coeliac disease. Gut. 2002; 50: 332–325. http://dx.doi.org/10.1136/gut.....
3.
Myszkowska-Ryciak J, Harton A, Gajewska D. Analysis of the nutritional value of the cost of a gluten-free diet compared to a standard food ration. Med Og Nauk Zdr. 2015; 21: 312–316. https://doi.org/10.5604/208345.... [in Polish].
4.
Rybicka I, Gliszczyńska-Świgło A. Nutrient deficiencies in a gluten-free diet. Probl Hig Epidemiol. 2016; 97: 183–186. [in Polish].
5.
Niland B, Cash BD. Health Benefits and Adverse Effects of a Gluten-Free Diet in Non-Celiac Disease Patients. Gastroenterol Hepatol (NY). 2018; 14(2): 82–91. PMC5866307.
6.
Moncayo R, Moncayo H. Exploring the aspect of psychosomatics in hypothyroidism: The WOMED model of body—mind interactions based on musculoskeletal changes, psychological stressors, and low levels of magnesium. Woman – Psychosom Gynaecol Obstet. 2014; 1: 1–11. http://dx.doi.org/10.1016/j.wo....
7.
Moncayo R, Moncayo H. The WOMED model of benign thyroid disease: Acquired magnesium deficiency due to physical and psychological stressors relates to dysfunction of oxidative phosphorylation. BBA Clin. 2014; 3: 44–64. https://doi.org/10.1016/j.bbac....
8.
Moncayo R, Moncayo H. Proof of concept of the WOMED model of benign thyroid disease: Restitution of thyroid morphology after correction of physical and psychological stressors and magnesium supplementation. BBA Clin. 2014; 3: 113–122. https://doi.org/10.1016/j.bbac....
9.
Ihnatowicz P, Drywień M, Wątor P, Wojsiat J. The importance of nutritional factors and dietary management of Hashimoto’s thyroiditis. Ann Agric Environ Med. 2020; 27(2): 184–193. https://doi.org/10.26444/aaem/....
10.
Schomburg L. Selenium, selenoproteins and the thyroid gland: interactions in health and disease. Nat Rev Endocrinol. 2011; 8(3): 160–171. doi: 10.1038/nrendo.2011.174.
11.
Wu Q, Rayman MP, Lv H, et al. Low Population Selenium Status Is Associated With Increased Prevalence of Thyroid Disease. J Clin Endocrinol Metab. 2015; 100(11): 4037–4047. doi: 10.1210/jc.2015-2222.
12.
Laurberg P, Andersen S, Pedersen IB, Knudsen N, Carlé A. Prevention of autoimmune hypothyroidism by modifying iodine intake and the use of tobacco and alcohol is manoeuvring between Scylla and Charybdis. Hormones (Athens). 2013; 12(1): 30–38. doi: 10.1007/BF03401284.
13.
Carlé A, Pedersen IB, Knudsen N, et al. Moderate alcohol consumption may protect against overt autoimmune hypothyroidism: a population-based case-control study. Eur J Endocrinol. 2012; 167(4): 483–490. doi: 10.1530/EJE-12-0356.
14.
Adadi P, Barakova NV, Muravyov KY, Krivoshapkina EF. Designing selenium functional foods and beverages: A review. Food Res Int. 2019; 120: 708–725. doi: 10.1016/j.foodres.2018.11.029.
15.
Naiyer AJ, Shah J, Hernandez L, Kim SY, Ciaccio EJ, Cheng J, Manavalan S, Bhagat G, Green PH. Tissue transglutaminase antibodies in individuals with celiac disease bind to thyroid follicles and extracellular matrix and may contribute to thyroid dysfunction. Thyroid 2008; 18: 1171–1178. https://doi.org/10.1089/thy.20....
16.
Roy A, Laszkowska M, Sundström J, Lebwohl B, Green PH, Kämpe O, Ludvigsson JF. Prevalence of Celiac Disease in Patients with Autoimmune Thyroid Disease: A Meta-Analysis. Thyroid 2016; 26: 880–890. https://doi.org/10.1089/thy.20....
17.
Husby S, Koletzko S, Korponay-Szabó IR, Mearin ML, Phillips A, Shamir R, et al. ESPGHAN Working Group on Coeliac Disease Diagnosis; ESPGHAN Gastroenterology Committee; European Society for Pediatric Gastroenterology, Hepatology, and Nutrition. European Society for Pediatric Gastroenterology, Hepatology, and Nutrition guidelines for the diagnosis of coeliac disease. J Pediatr Gastroenterol Nutr. 2012; 54: 136–160. https://doi.org/10.1097/MPG.0b....
18.
Sari S, Yesilkaya E, Egritas O, Bideci A, Dalgic B. Prevalence of celiac disease in Turkish children with autoimmune thyroiditis. Dig Dis Sci. 2009; 54: 830–832. https://doi.org/10.1007/s10620....
19.
Marwaha RK, Garg MK, Tandon N, Kanwar R, Narang A, Sastry A, Saberwal A, Bhadra K. Glutamic acid decarboxylase (anti-GAD) &tissue transglutaminase (anti-TTG) antibodies in patients with thyroid autoimmunity. Indian J Med Res. 2013; 137: 82–86.
20.
Guliter S, Yakaryilmaz F, Ozkurt Z, Ersoy R, Ucardag D, Atasoy P. Prevalence of coeliac disease in patients with autoimmune thyroiditis in a Turkish population. World J Gastroenterol. 2007; 13: 1599–1601.
21.
Riseh SH, Farhang AM, Mobasser M, Jafarabadi AM. The Relationship between Thyroid Hormones, Antithyroid Antibodies, Anti-Tissue Transglutaminase and Anti-Gliadin Antibodies in Patients with Hashimoto’s Thyroiditis. Acta Endocrinol (Buchar). 2017; 13: 174–179. https://doi.org/10.4183/aeb.20....
22.
Kowalska E, Wasowska-Królikowska K, Toporowska-Kowalska E. Estimation of antithyroid antibodies occurrence in children with coeliac disease. Med Sci Monit. 2000; 6: 719–721.
23.
Meloni A, Mandas C, Jores RD, Congia M. Prevalence of autoimmune thyroiditis in children with celiac disease and effect of gluten withdrawal. J Pediatr. 2009; 155: 51–55, 55.e1. https://doi.org/10.1016/j.jped....
24.
Hakanen M, Luotola K, Salmi J, Laippala P, Kaukinen K, Collin P. Clinical and subclinical autoimmune thyroid disease in adult celiac disease. Dig Dis Sci. 2001; 46: 2631–2635.
25.
Velluzzi F, Caradonna A, Boy MF, Pinna MA, Cabula R, Lai MA, Piras E, Corda G, Mossa P, Atzeni F, et al. Thyroid and celiac disease: clinical, serological, and echographic study. Am J Gastroenterol. 1998; 93: 976–979.
26.
Carta MG, Hardoy MC, Boi MF, Mariotti S, Carpiniello B, Usai P. Association between panic disorder, major depressive disorder and celiac disease: a possible role of thyroid autoimmunity. J Psychosom Res 2002; 53: 789–793.
27.
Collin P, Reunala T, Pukkala E, Laippala P, Keyriläinen O, Pasternack A. Coeliac disease-associated disorders and survival. Gut 1994; 35: 1215–1218.
28.
Guariso G, Conte S, Presotto F, et al. Clinical, subclinical and potential autoimmune diseases in an Italian population of children with coeliac disease. Aliment Pharmacol Ther. 2007; 26(10): 1409–1417. doi: 10.1111/j.1365-2036.2007.03526.x.
29.
Kumar R, Saraf S. Autoimmune thyroiditis with fluctuating antibodies. Endocrine Abstracts. 2019; 62 CB7 | doi: 10.1530/endoabs.62.CB7.
30.
Kahaly GJ, Frommer L, Schuppan D. Celiac disease and endocrine autoimmunity – the genetic link. Autoimmun Rev. 2018; 17: 1169–1175. https://doi.org/10.1016/j.autr....
31.
Stazi AV, Trinti B. Selenium status and over-expression of interleukin-15 in celiac disease and autoimmune thyroid disease. Ann Ist Super Sanita. 2010; 46: 389–399. https://doi.org/10.4415/ANN_10....
32.
Ambroziak U, Hybsier S, Shahnazaryan U, Krasnodębska-Kiljańska M, Rijntjes E, Bartoszewicz Z, Bednarczuk T, Schomburg L. Severe selenium deficits in pregnant women irrespective of autoimmune thyroid disease in an area with marginal selenium intake. J Trace Elem Med Biol. 2017; 44: 186–191. https://doi.org/10.1016/j.jtem....
33.
Ihnatowicz P, Wątor P, Drywień ME. Supplementation in Autoimmune Thyroid Hashimoto’s Disease. Vitamin D and Selenium. J Food Nutr Res. 2019; 7: 584–591. https://doi.org/10.12691/jfnr-....
34.
Markiewicz-Zukowska R, Naliwajko SK, Bartosiuk E, Sawicka E, Omeljaniuk WJ, Borawska MH. Zawartość witamin w dietach kobiet z chorobą Hashimoto. Bromat Chem Toksykol. 2011, XLIV: 539–543.
35.
Krysiak, R, Szkróbka W, Okopień, B. The Effect of Gluten-Free Diet on Thyroid Autoimmunity in Drug-Naive Women with Hashimoto’s Thyroiditis: A Pilot Study. Exp Clin Endocrinol Diabetes. 2019; 127: 417–422. https://doi.org/10.1055/a-0653....
36.
Lizis-Kolus K. Ocena wpływu niedoboru witaminy D na przebieg choroby Hashimoto u chorych w województwie świętokrzyskim. Praca doktorska. Kraków: Uniwersytet Jagielloński; 2015. [in Polish].
37.
Krysiak R, Szkróbka W, Okopień B. The effect of vitamin D and selenomethionine on thyroid antibody titers, hypothalamic-pituitary-thyroid axis activity and thyroid function tests in men with Hashimoto’s thyroiditis: A pilot study. Pharmacol Rep. 2018; 71: 243–247. https://doi.org/10.1016/j.phar....
38.
Assa A, Frenkel-Nir Y, Tzur D, et al. Large population study shows that adolescents with celiac disease have an increased risk of multiple autoimmune and nonautoimmune comorbidities. Acta Paediatr. 2017; 106: 967–972. https://doi.org/10.1111/apa.13....
39.
Hess SY, Zimmermann MB, Arnold M, Langhans W, Hurrell RF. Iron deficiency anemia reduces thyroid peroxidase activity in rats. J Nutr. 2002; 132(7): 1951–5. PMID: 12097675. https://doi.org/10.1093/jn/132....
40.
Dahiya K, Verma M, Dhankhar R, et al. Thyroid profile and iron metabolism: mutual relationship in hypothyroidism. Biomed Res (India). 2016; 27: 1212–1215.
41.
Wopereis DM, Du Puy RS, van Heemst D, et al. The Relation Between Thyroid Function and Anemia: A Pooled Analysis of Individual Participant Data. J Clin Endocrinol Metab. 2018; 103: 3658–3667. https://doi.org/10.1210/jc.201....
42.
Khatiwada S, Gelal B, Baral N, et al. Association between iron status and thyroid function in Nepalese children. Thyroid Res. 2016; 9: 2. https://doi.org/10.1186/s13044....
43.
Shukla A, Agarwal S, Gupta A, et al. Relationship between Body Iron Status and Thyroid Profile in an Adult Population: A Hospital Based Study. Natl Lab Med. 2017; 6: BO01-BO03. https://doi.org/10.7860/NJLM/2....
44.
Rooks MG, Garrett WS. Gut microbiota, metabolites and host immunity. Nat Rev Immunol. 2016; 16: 341–352. https://doi.org/10.1038/nri.20....
45.
Zhang J, Zhang F, Zhao C, et al. Dysbiosis of the gut microbiome is associated with thyroid cancer and thyroid nodules and correlated with clinical index of thyroid function. Endocrine. 2019; 64: 564–574. https://doi.org/10.1007/s12020....
46.
Ishaq HM, Mohammad IS, Guo H, et al. Molecular estimation of alteration in intestinal microbial composition in Hashimoto’s thyroiditis patients. Biomed Pharmacother. 2017; 95: 865–874. https://doi.org/10.1016/j.biop....
47.
Virili C, Fallahi P, Antonelli A, et al. Gut microbiota and Hashimoto’s thyroiditis. Rev Endocr Metab Disord. 2018; 19: 293–300. https://doi.org/10.1007/s11154....
48.
Fasano A. Zonulin and its regulation of intestinal barrier function: the biological door to inflammation, autoimmunity, and cancer. Physiol Rev. 2011 Jan; 91(1): 151–75. doi: 10.1152/physrev.00003.2008. PMID: 21248165.
49.
Küçükemre Aydin B, Yildiz M, Akgün A, et al. Children with Hashimoto’s Thyroiditis Have Increased Intestinal Permeability: Results of a Pilot Study. J Clin Res Pediatr Endocrinol. Epub. 2020; 12(3): 303–307. https://doi.org/10.4274/jcrpe.....
50.
Fasano, A. Intestinal Permeability and its Regulation by Zonulin: Diagnostic and Therapeutic Implications. Clin Gastroenterol Hepatol. 2012; 10: 1096-1100. https://doi.org/10.1016/j.cgh.....
51.
Vancamelbeke M, Vermeire S. The intestinal barrier: a fundamental role in health and disease. Expert Rev Gastroenterol Hepatol. 2017; 11: 821–834. https://doi.org/10.1080/174741....
52.
Twito O, Shapiro Y, Golan-Cohen A. et al. Anti-thyroid antibodies, parietal cell antibodies and tissue transglutaminase antibodies in patients with autoimmune thyroid disease. Arch Med Sci. 2018; 14: 516–520. https://doi.org/10.5114/aoms.2....
53.
Rose NR. Insight into Mechanisms of Autoimmune Disease Based on Clinical Findings. In: Sudhir P, editor. Autoimmune Reactions. Totowa, New Jersey: Humana Press; 1999. p. 5–14. https://doi.org/10.1007/978-1-....
54.
Siomkajło M, Dybko J, Daroszewski J. Regulatory lymphocytes in thyroid orbitopathy and autoimmune thyroid diseases. Postepy Hig Med Dosw. (online). 2016; 70(0): 1378–1388. https://doi.org/10.5604/173226....
55.
Theofilopoulos AN, Kono DH, Baccala R. The multiple pathways to autoimmunity. Nat Immunol. 2017; 18(7): 716–724. https://doi.org/10.1038/ni.373....
56.
Szaflarska A, Rutkowska-Zapała M, Kowalczyk D. Immune tolerance mechanisms – brief review. Przegląd Lekarski. 2015; 72(12): 765–769.
57.
Paul S. Diversity of Immunological Defects in Autoimmune Diseases. In: Paul S, editor. Autoimmune Reactions. Totowa, New Jersey: Humana Press; 1999. p. 1–4. https://doi.org/10.1007/978-1-....
58.
Peng S, Li C, Wang X, et al. Increased Toll-Like Receptors Activity and TLR Ligands in Patients with Autoimmune Thyroid Diseases. Front Immunol. 2016; 7: 578. https://doi.org/10.3389/fimmu.....
59.
Worthington J, Silman AJ. Genetic control of autoimmunity, lessons from twin studies. Clin Exp Immunol. 1995; 101(3): 390–392. https://doi.org/10.1111/j.1365....
60.
Brix TH, Hegedüs L. Twin studies as a model for exploring the aetiology of autoimmune thyroid disease. Clin Endocrinol (Oxf). 2012; 76(4): 457–64. https://doi.org/10.1111/j.1365....
61.
Rochester JR, Bolden AL. Bisphenol S and F: A Systematic Review and Comparison of the Hormonal Activity of Bisphenol A Substitutes. Environ Health Perspect. 2015; 123(7): 643–50. https://doi.org/10.1289/ehp.14....
62.
Benvenga S, Elia G, Ragusa F, et al. Endocrine disruptors and thyroid autoimmunity. Best Pract Res Clin Endocrinol Metab. 2020; 34(1): 101377. https://doi.org/10.1289/ehp.14....
63.
Ferrari SM, Fallahi P, Antonelli A, Benvenga S. Environmental Issues in Thyroid Diseases. Front Endocrinol. 2017; 8: 50. https://doi.org/10.3389/fendo.....
64.
Aaron L, Torsten M, Patricia W. Autoimmunity in celiac disease: Extra-intestinal manifestations. Autoimmun Rev. 2019; 18(3): 241–246. https://doi.org/10.1016/j.autr....
65.
Ventura A, Neri E, Ughi C, et al. Gluten-dependent diabetes-related and thyroid-related autoantibodies in patients with celiac disease. J Pediatr. 2000; 137: 263–265. https://doi.org/10.1067/mpd.20....
66.
Oderda G, Rapa A, Zavallone A, et al. Thyroid autoimmunity in childhood celiac disease. J Pediatr Gastroenterol Nutr. 2002; 35: 704–705. https://doi.org/10.1097/000051....
67.
Toscano V, Conti FG, Anastasi E, et al. Importance of gluten in the induction of endocrine autoantibodies and organ dysfunction in adolescent celiac patients. Am J Gastroenterol. 2000; 95: 1742–1748. https://10.1111/j.1572-0241.20....
68.
Magazzu G, De Luca F, Benvenga S, et al. Hypothyroidie biologique transitoire chez un nourrisson porteur d’une maladie coeliaque. Pediatrie 1983; 38(4): 249–252.
69.
Cosnes J, Cellier C, Viola S, et al. Groupe D’Etude et de Recherche Sur la MaladieCoeliaque. Incidence of autoimmune diseases in celiac disease: protective effect of the gluten-free diet. Clin Gastroenterol Hepatol. 2008; 6(7): 753–8. https://doi.org/10.1016/j.cgh.....
70.
Ansaldi, N, Palmas T, Corrias A, et al. Autoimmune thyroid disease and celiac disease in children. J Pediatr Gastroenterol Nutr. 2003; 37(1): 63–6. https://doi.org/10.1097/000051....
71.
Diamanti A, Ferretti F, Guglielmi R, et al. Thyroid autoimmunity in children with coeliac disease: a prospective survey. Arch Dis Child. 2011; 96(11): 1038–41. https://doi.org/10.1136/archdi....
72.
Metso S, Hyytiä-Ilmonen H, Kaukinen K, et al. Gluten-free diet and autoimmune thyroiditis in patients with celiac disease. A prospective controlled study. Scand J Gastroenterol. 2012; 47(1): 43–8. https://doi.org/10.3109/003655....
73.
Minelli R, Gaiani F, Kayali S, et al. Thyroid and celiac disease in pediatric age: a literature review. Acta Biomed. 2018; 89(9-S): 11–16. https://doi.org/10.23750/abm.v....
74.
Cassio A, Ricci G, Baronio F, et al. Long-term clinical significance of thyroid autoimmunity in children with celiac disease. J Pediatr. 2010; 156(2): 292–295. https://doi.org/10.1016/j.jped....
75.
Sategna-Guidetti C, Volta U, Ciacci C, Usai P, Carlino A, De Franceschi L, Camera A, Pelli A, Brossa C. Prevalence of thyroid disorders in untreated adult celiac disease patients and effect of gluten withdrawal: an Italian multicenter study. Am J Gastroenterol. 2001; 96(3): 751–7. https://doi.org/10.1111/j.1572....
76.
Rasheed J, Hassan R, Khalid M, et al. Frequency of autoimmune thyroiditis in children with Celiac disease and effect of gluten free diet. Pak J Med Sci. 2020; 36(6): 1280–1284. https://doi.org/10.12669/pjms.....
77.
Krysiak R, Kowalcze K, Okopien B. The effect of vitamin D on thyroid autoimmunity in non-lactating women with postpartum thyroiditis. Eur J Clin Nutr. 2016; 70(5): 637–9. https://doi.org/10.1038/ejcn.2....
78.
Krysiak R, Okopien B. The effect of levothyroxine and selenomethionine on lymphocyte and monocyte cytokine release in women with Hashimoto’s thyroiditis. J Clin Endocrinol Metab. 2011; 96(7): 2206–15. https://doi.org/10.1210/jc.201....
79.
Krysiak R, Kowalcze K, Okopien B. Selenomethionine potentiates the impact of vitamin D on thyroid autoimmunity in euthyroid women with Hashimoto’s thyroiditis and low vitamin D status. Pharmacol Rep. 2019; 71(2): 367–373. https://doi.org/10.1016/j.phar....
80.
Kłapcińska B, Poprzecki S, Danch A, et al. Selenium levels in blood of Upper Silesian population: evidence of suboptimal selenium status in a significant percentage of the population. Biol Trace Elem Res. 2005; 108(1–3): 1–15. https://doi.org/10.1385/BTER:1....
81.
Antvorskov JC, Fundova P, Buschard K, et al. Dietary gluten alters the balance of pro-inflammatory and anti-inflammatory cytokines in T cells of BALB/c mice. Immunology. 2013; 138(1): 23–33. https://doi.org/10.1111/imm.12....
82.
Street ME, Volta C, Ziveri MA, et al. Changes and relationships of IGFS and IGFBPS and cytokines in coeliac disease at diagnosis and on gluten-free diet. Clin Endocrinol (Oxf). 2008; 68(1): 22–28. http://doi.org/10.1111/j.1365-....
83.
Arrigo T, Wasniewska M, Crisafulli G, et al. Subclinical hypothyroidism: the state of the art. J Endocrinol Invest. 2008; 31(1): 79–84. https://doi.org/.10.1007-BF033....
84.
Chaker L, Bianco AC, Jonklaas J, et al. Hypothyroidism. Lancet. 2017; 390: 1550–1562. https://doi.org/10.1016/S0140-....
85.
Asimi ZV, Hadzovic-Dzuvo A, Tawil DA. The effect of selenium supplementation and gluten-free diet in patients with subclinical hypothyroidism affected by autoimmune thyroiditis. Endocrine Abstracts. 2020; 70: AEP906. https://doi.org/10.1530/endoab....
86.
Avard N, Grand SJ. A case report of a novel, integrative approach to Hashimoto’s thyroiditis with unexpected results. Adv Integr Med. 2018; 5(2): 75–79. https:/doi.org/10.1016/j.aimed.2018.03.003.
87.
Seshadri D, De D. Nails in nutritional deficiencies. Indian J Dermatol Venereol Leprol. 2012; 78(3): 237–41. https://doi.org/10.4103/0378-6....
88.
Coppede F. One-carbon metabolism and Alzheimer’s disease: focus on epigenetics. Curr Genomics. 2010; 11(4): 246–60. https://doi.org/10.2174/138920....
89.
Greer JM, McCombe PA. The role of epigenetic mechanisms and processes in autoimmune disorders. Biologics. 2012; 6: 307–27. https://doi.org/10.2147/BTT.S2....
90.
Arakawa Y, Watanabe M, Inoue N, et al. Association of polymorphisms in DNMT1, DNMT3A, DNMT3B, MTHFR and MTRR genes with global DNA methylation levels and prognosis of autoimmune thyroid disease. Clin Exp Immunol. 2012; 170(2): 194–201. https://doi.org/10.1111/j.1365....
91.
Cai TT, Zhang J, Wang X, et al. Gene-gene and gene-sex epistatic interactions of DNMT1, DNMT3A and DNMT3B in autoimmune thyroid disease. Endocr J. 2016; 30; 63(7): 643–653. https://doi.org/10.1507/endocr....
92.
Suarez-Alvarez B, Rodriguez RM, Fraga MF, et al. DNA methylation: a promising landscape for immune system-related diseases. Trends Genet. 2012; 28(10): 506–14. https://doi.org/10.1016/j.tig.....
93.
Mu Q, Kirby J, Reilly CM, et al. Leaky Gut As a Danger Signal for Autoimmune Diseases. Front Immunol. 2017; 8: 598. https://doi.org/10.3389/fimmu.....
94.
Higuchi BS, Rodrigues N, Gonzaga MI, et al. Intestinal Dysbiosis in Autoimmune Diabetes Is Correlated With Poor Glycemic Control and Increased Interleukin-6: A Pilot Study. Front Immunol. 2018; 9: 1689. https://doi.org/10.3389/fimmu.....
95.
Mirhosseini N, Brunel L, Muscogiuri G, et al. Physiological serum 25-hydroxyvitamin D concentrations are associated with improved thyroid function-observations from a community-based program. Endocrine. 2017; 58(3): 563–573. https://doi.org/10.1007/s12020....
96.
Abbott RD, Sadowski A, Alt AG. Efficacy of the Autoimmune Protocol Diet as Part of a Multi-disciplinary, Supported Lifestyle Intervention for Hashimoto’s Thyroiditis. Cureus. 2019; 11(4): e4556. https://doi.org/10.7759/cureus....
97.
Konijeti GG, Kim N, Lewis JD, et al. Efficacy of the Autoimmune Protocol Diet for Inflammatory Bowel Disease. Inflamm Bowel Dis. 2017; 23(11): 2054–2060. https://doi.org/10.1097/MIB.00....
98.
Winther KH, Cramon P, Watt T, et al. Disease-Specific as Well as Generic Quality of Life Is Widely Impacted in Autoimmune Hypothyroidism and Improves during the First Six Months of Levothyroxine Therapy. PLoS One. 2016; 11(6): e0156925. https://doi.org/10.1371/journa....
99.
Valentino R, Savastano S, Tomaselli AP. Unusual association of thyroiditis, Addison’s disease, ovarian failure and celiac disease in a young woman. J Endocrinol Invest. 1999; 22(5): 390–4. https:/doi.org/10.1007/BF03343578.
100.
Moncayo R, Moncayo H. Applying a systems approach to thyroid physiology: Looking at the whole with a mitochondrial perspective instead of judging single TSH values or why we should know more about mitochondria to understand metabolism. BBA Clin. 2017; 7: 127–140. https://doi.org/10.1016/j.bbac....
101.
Farahid OH, Khawaja N, Shennak MM, et al. Prevalence of coeliac disease among adult patients with autoimmune hypothyroidism in Jordan. East Mediterr Health J. 2014; 20(1): 51–5.
102.
Teixeira LM, Nisihara R, da Rosa Utiyama SR, et al. Screening of celiac disease in patients with autoimmune thyroid disease from Southern Brazil. Arq Bras Endocrinol Metab. 2014; 58(6). http://dx.doi.org/10.1590/0004....
103.
Zubarik R, Ganguly E, Nathan M, Vecchio J. Celiac disease detection in hypothyroid patients requiring elevated thyroid supplementation: A prospective cohort study. Eur J Intern Med. 2015; 26(10): 825–9. https://doi.org/10.1016/j.ejim....
104.
Tuhan H, Işik S, Abaci A, et al. Celiac disease in children and adolescents with Hashimoto Thyroiditis. Turk Pediatri Ars. 2016; 51(2): 100–5. https://doi.org/10.5152/TurkPe....
105.
Gil M, Aparicio MM, Ercoli V, et al. Prevalence of celiac disease in autoimmune thyroid diseases, and its associaton with otherautoimmune disesases: a single center study in Argentina. Endocrine Abstracts. 2019; 63: P38. https://doi.org/10.1530/endoab....
106.
Van der Pals M, Ivarsson A, Norström F, Högberg L, Svensson J, Carlsson A. Prevalence of thyroid autoimmunity in children with celiac disease compared to healthy 12-year olds. Autoimmune Dis. 2014; 2014: 417356. https://doi.org/10.1155/2014/4....
107.
Kalyoncu D, Urganci N. Antithyroid antibodies and thyroid function in pediatric patients with celiac disease. Int J Endocrinol. 2015; 2015: 276575. https://doi.org/10.1155/2015/2....
108.
Baharvand P, Hormozi M, Aaliehpour A. Comparison of thyroid disease prevalence in patients with celiac disease and controls. Gastroenterol Hepatol Bed Bench. 2020; 13(1): 44–49.
109.
Rasheed J, Hassan R, Khalid M, Zafar F. Frequency of autoimmune thyroiditis in children with Celiac disease and effect of gluten free diet. Pak J Med Sci. 2020; 36(6): 1280–1284. https://doi.org/10.12669/pjms.....
110.
Mitrogiorgou M, Karachaliou F, Karalexi M, et al. Celiac disease and endocrine autoimmunity in children and adolescents. ESPE Abstracts. 2019; 92: P2–214.
111.
Gupta V, Singh A, Khadgawat R, et al. The spectrum of clinical and subclinical endocrinopathies in treatment-naive patients with celiac disease. Indian J Gastroenterol. 2019; 38(6): 518–526. https://doi.org/10.1007/s12664....
112.
Tiberti C, Panimolle F, Borghini R, et al. Type 1 diabetes, thyroid, gastric and adrenal humoral autoantibodies are present altogether in almost one third of adult celiac patients at diagnosis, with a higher frequency than children and adolescent celiac patients. Scand J Gastroenterol. 2020; 55(5): 549–554. https://doi.org/10.1080/003655....
113.
Şahin, Şükrü, Şahin FD. Autoimmune Thyroid Disease, Thyroid Functions, and Thyroid Ultrasonography in Pediatric Celiac Disease. Med Sci Discover. 2020; 7(11): 680–683. https://doi.org/10.36472/msd.v....
114.
Khalaf BS. Association of Autoimmune Thyroiditis and Type 1 Diabetes Mellitus With Severity of Children with Celiac Disease. Indian J Forensic Med Toxicol. 2020; 14(1). https://doi.org/10.37506/v14/i....
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.