Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
RESEARCH PAPER
Quality of life of patients undergoing specific allergen immunotherapy in allergic rhinitis
1
Medical University, Lublin, Poland
2
University of Economics and Innovation, Department of Human Science, Lublin, Poland
3
Center of Allergology, Lublin, Poland
4
University of Radom, Poland
5
Institute of Rural Health, Lublin, Poland
6
Department of Medical Anthropology, Institute of Rural Health, Lublin, Poland
7
Department of Epidemiology and Biostatistics, Institute of Rural Health, Lublin, Poland
Corresponding author
Magdalena Brodowicz-Król
Uniwersytet Medyczny w Lublinie, Prosesora Antoniego Gębali 6, 20-093, Lublin, Poland
Uniwersytet Medyczny w Lublinie, Prosesora Antoniego Gębali 6, 20-093, Lublin, Poland
Ann Agric Environ Med. 2020;27(4):657-663
KEYWORDS
TOPICS
ABSTRACT
Introduction:
Although allergic diseases have been known since antiquity, both their understanding and systematics came later. The World Allergy Organization (WAO) defines the phenomenon of atopy as a predisposition of a person or family to the uncontrolled synthesis and release of IgE antibodies. Allergic rhinitis (AR) is one of the most important clinical diseases of rhinitis (NN, rhinitis). AR significantly reduces the quality of life, tends to increase, and its consequences may be life-threatening diseases.
Objective:
The aim of the study is to determine the quality of life of patients who underwent specific allergen immunotherapy in allergic rhinitis.
Material and methods:
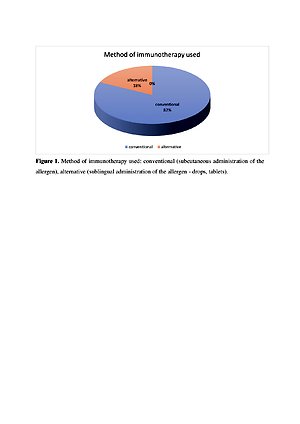
The study was conducted at the Center for Specialist Allergology in Lublin between October 2018 – February 2019. The study covered a group of 157 patients. The diagnostic method used was a questionnaire. The first research tool was own questionnaire consisting of 31 questions. The second tool was the standardized questionnaire, the Polish version of the SF-36 Quality of Life Questionnaire.
Results:
Studies have shown statistically significant changes in the symptoms of before and after immunotherapy, which means improving the quality of life and reducing the severity of symptoms and problems of respondents after therapy. The respondents were not in the best of health (50%). Over 50% of respondents said that the immunotherapy process significantly reduced personal / family expenses for treatment associated with allergic rhinitis.
Conclusions:
The conducted process of specific allergen immunotherapy improved the quality of life of respondents by increasing awareness of the quality of life through the prism of health change in relation to the general indicator in the area of mental problems by reducing their nuisance value more than in the area of somatic symptoms.
Although allergic diseases have been known since antiquity, both their understanding and systematics came later. The World Allergy Organization (WAO) defines the phenomenon of atopy as a predisposition of a person or family to the uncontrolled synthesis and release of IgE antibodies. Allergic rhinitis (AR) is one of the most important clinical diseases of rhinitis (NN, rhinitis). AR significantly reduces the quality of life, tends to increase, and its consequences may be life-threatening diseases.
Objective:
The aim of the study is to determine the quality of life of patients who underwent specific allergen immunotherapy in allergic rhinitis.
Material and methods:
The study was conducted at the Center for Specialist Allergology in Lublin between October 2018 – February 2019. The study covered a group of 157 patients. The diagnostic method used was a questionnaire. The first research tool was own questionnaire consisting of 31 questions. The second tool was the standardized questionnaire, the Polish version of the SF-36 Quality of Life Questionnaire.
Results:
Studies have shown statistically significant changes in the symptoms of before and after immunotherapy, which means improving the quality of life and reducing the severity of symptoms and problems of respondents after therapy. The respondents were not in the best of health (50%). Over 50% of respondents said that the immunotherapy process significantly reduced personal / family expenses for treatment associated with allergic rhinitis.
Conclusions:
The conducted process of specific allergen immunotherapy improved the quality of life of respondents by increasing awareness of the quality of life through the prism of health change in relation to the general indicator in the area of mental problems by reducing their nuisance value more than in the area of somatic symptoms.
Brodowicz-Król M, Guz E, Hawryluk D, Kulbaka E, Panasiuk L, Piotr Lutomski, Paulina Kaczor-Szkodny, Piotr Choina. Quality of life of patients
undergoing specific allergen immunotherapy in allergic rhinitis. Ann Agric Environ Med. 2020; 27(4): 657–663. doi: 10.26444/aaem/127840
REFERENCES (30)
1.
Rogala B, Glück J. Immunoterapia alergenowa, ed. Kowalski ML, Rogala B. Łódź: MEDITON; 2012.
2.
Seidman MD, Gurgel RK, Lin SY, et al. Clinical practice guideline: Allergic rhinitis. Otolaryngol Head Neck Surg. 2015 Feb; 152(1 Suppl): S1–43. doi: 10.1177/0194599814561600.
3.
Meltzer EO. Allergic Rhinitis: Burden of Illness, Quality of Life, Comorbidities, and Control. Immunol Allergy Clin North Am. 2016; 36(2): 235–248. doi:10.1016/j.iac.2015.12.002.
4.
Hoyte FCL, Nelson HS. Recent advances in allergic rhinitis. F1000Res. 2018; 7: F1000 Faculty Rev-1333. Published 2018 Aug 23. doi:10.12688/f1000research.15367.1.
5.
Kakli HA, Riley TD. Allergic Rhinitis. Prim Care. 2016 Sep; 43(3): 465–75. doi: 10.1016/j.pop.2016.04.009.
6.
Stanisławska M. Jakość życia pacjentów z alergicznym niezytem nosa. Fam Med Prim Care Rev. 2015; 17: 3.
7.
Okubo K, Kurono Y, Ichimura K, et al. Japanese guidelines for allergic rhinitis 2020 [published online ahead of print, 2020 May 27]. Allergol Int. 2020; S1323–8930(20)30050–2. doi: 10.1016/j.alit.2020.04.001.
8.
Emeryk A, Rapiejko P, Lipiec A. Alergiczny nieżyt nosa – kompendium dla lekarzy. Poznań: Wyd. Medyczne Termedia; 2013. p. 25–38.
9.
Chazan R. Pneumonologia i alergologia – badania diagnostyczne i postępowanie? – medica press, 2010/2011, 119–122.
10.
Kowalski L. Przedmowa do wydania I. In: Immunoterapia alergenowa; ed. Kowalski M, Rogala B. Łódź: MEDITON; 2012.
11.
Rogala B. Komentarz. Postępowanie w alergicznym nieżycie nosa. Podsumowanie wytycznych Allergic Rhinitis and its Impact on Asthma. Med Prakt. 2018; 4: 57.
12.
Konradsen J, Arvidsson M. Allergen-specific immunotherapy provides long-lasting symptom relief. Lakartidningen. 2016 Apr 4; 113: DW74.
13.
Allam JP, Novak N. Immunologische Mechanismen der allergen-spezifischen Immuntherapie [Immunological mechanisms of allergen-specific immunotherapy]. Hautarzt. 2017; 68(4): 265–270. doi: 10.1007/s00105-017-3961-0.
14.
Głobińska A, Boonpiyathad T, Satitsuksanoa P, et al. Mechanisms of allergen-specific immunotherapy: Diverse mechanisms of immune tolerance to allergens. Ann Allergy Asthma Immunol. 2018; 121(3): 306–312. doi: 10.1016/j.anai.2018.06.026.
15.
Roberts G, Pfaar O, Akdis CA, et al. EAACI Guidelines on Allergen Immunotherapy: Allergic rhinoconjunctivitis. Allergy. 2018; 73(4): 765–798. doi:10.1111/all.13317.
16.
Samoliński B, Arcimowicz M, Polskie Standardy Leczenia Nieżytów Nosa. Alergologia Polska 2013.
18.
Gocki J, Bartuzi Z. Wytyczne/zalecenia. Podskórna i podjęzykowa droga stosowania immunoterapii alergenowej. Schematy leczenia. In: Alergologia Polska. Polish J Allergol. 2018; 5: 137–144.
19.
Bartkowiak-Emeryk M. Immunoterapia alergenowa w alergicznym nieżycie nosa. In: Post Dermatol Alergol. 2012: 32–38.
20.
Dobrzańska A, Ryżko J, Pediatria, podręcznik do Lekarskiego Egzaminu Końcowego i Państwowego Egzaminu Specjalizacyjnego, ed. Dobrzańska A, Ryżko R. Wrocław: ELSEVIER Urban &Partner; 2014.
21.
Cichocka-Jarosz E. Influence of allergen immunotherapy on the quality of life in patients with asthma, allergic rhinitis and insect venom allergy. Alergol Pol – Polish J Allergol. 2018; 5(3): 148–156. doi: 10.5114/pja.2018.78595.
22.
Zawadzka-Krajewska A. Alergiczny nieżyt nosa. In: Pediatria, podręcznik do Lekarskiego Egzaminu Końcowego i Państwowego Egzaminu Specjalizacyjnego, ed. Dobrzańska A, Ryżko R. Wrocław: ELSEVIER Urban &Partner; 2014.
23.
Halken S, Larenas-Linnemann D, Roberts G, et al. EAACI guidelines on allergen immunotherapy: Prev Allergy. Pediatr Allergy Immunol. 2017; 28(8): 728–745. doi: 10.1111/pai.12807.
24.
Nelson HS, Calderon MA, Bernstein DI, et al. Allergen immunotherapy clinical trial outcomes and design: working toward harmonization of methods and principles. Curr Allergy Asthma Rep. 2017 Mar; 17(3): 18. doi: 10.1007/s11882-017-0687-0.
25.
Petersen KD, Kronborg C, Larsen JN, Dahl R, Gyrd-Hansen D. Patient related outcomes in a real life prospective follow up study: Allergen immunotherapy increase quality of life and reduce sick days. World Allergy Organ J. 2013; 6(1): 15. Published 2013 Sep 9. doi:10.1186/1939-4551-6-15.
26.
Roger A, Arcalá Campillo E, Torres MC, et al. Reduced work/academic performance and quality of life in patients with allergic rhinitis and impact of allergen immunotherapy. Allergy Asthma Clin Immunol. 2016; 12: 40. https://doi.org/10.1186/s13223....
27.
Filanowicz M, Szynkiewicz E, Cegła B, Bartuzi Z. Analiza jakości życia pacjentów z astmą i alergicznym nieżytem nosa po immunoterapii. Dermatol Alergol. 2016; 33: 134–41.
28.
Modrzyński M, Rapiejko P, Zawisza E. Ocena jakości życia chorych z alergicznym nieżytem nosa leczonych za pomocą immunoterapii swoistej. Ann Univ Mariae Curie Sklodowska 2005; 60 (16), 328: 471–475.
29.
Lin SY, Erekosima N, Suarez-Cuervo C, et al. Allergen-Specific Immunotherapy for the Treatment of Allergic Rhinoconjunctivitis and/or Asthma: Comparative Effectiveness Review [Internet]. Rockville (MD): Agency for Healthcare Research and Quality (US); 2013 Mar. Report No.: 13-EHC061-EF. PMID: 23638484.
30.
Meadows A, Kaambwa B, Novielli N, et al. A systematic review and economic evaluation of subcutaneous and sublingual allergen immunotherapy in adults and children with seasonal allergic rhinitis. Health Technol Assess. 2013 Jul; 17(27): vi, xi-xiv, 1–322. doi: 10.3310/hta17270.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.