Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
RESEARCH PAPER
Impact of phytochemicals and plant extracts on viability and proliferation of NK cell line NK-92 – a closer look at immunomodulatory properties of goji berries extract in human colon cancer cells
1
Department of Experimental Hematooncology, Medical University, Lublin, Poland
2
Department of Medical Biology, Institute of Rural Health, Lublin, Poland
3
Department of Functional Anatomy and Cytobiology, Maria Curie-Skłodowska University, Lublin, Poland
Corresponding author
Ann Agric Environ Med. 2021;28(2):291-299
KEYWORDS
TOPICS
ABSTRACT
Introduction:
Due to the fact that lymphocytes NK (natural killer cells) are the first line of defence of the body against cancer, one of the goals of modern immunotherapy is the enhancement of their natural activities for the effective recognition, detection, and elimination of cancer cells.
Objective:
The aim of the study was to evaluate the influence of selected phytochemicals (curcumin and resveratrol) and plant extracts (chlorella and goji berries) on NK cells viability and proliferation, as well as cytotoxic activity against colon cancer – one of the most common cancer worldwide.
Material and methods:
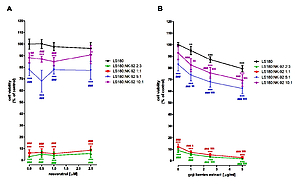
The impact of phytochemicals, viability and proliferation of plant extracts on NK cells was examined in NK-92 cells using both LDH and MTT assays. The immunomodulatory properties of selected compounds were tested against human colon cancer cell line LS180 using the MTT test.
Results:
Extracts of chlorella and goji berries significantly increased NK cell proliferation, while curcumin and resveratrol did not affect this process. Curcumin, as well as extracts of chlorella and goji berries, did not impact NK viability, while resveratrol significantly increased it. LDH test revealed the cytotoxic effect of chlorella extract and curcumin in NK-92 cell cultures. On the contrary, goji berries extract significantly decreased LDH level, while resveratrol did not affect the integrity of NK cell membranes. Studies conducted in co-cultures NK cells, also directly eliminated colon cancer cells.
Conclusions:
Performed studies revealed immunomodulatory properties of goji berries extract, which improved viability and proliferation of NK cells, and above all, significantly increased their ability to recognize and eliminate colon cancer cells.
Due to the fact that lymphocytes NK (natural killer cells) are the first line of defence of the body against cancer, one of the goals of modern immunotherapy is the enhancement of their natural activities for the effective recognition, detection, and elimination of cancer cells.
Objective:
The aim of the study was to evaluate the influence of selected phytochemicals (curcumin and resveratrol) and plant extracts (chlorella and goji berries) on NK cells viability and proliferation, as well as cytotoxic activity against colon cancer – one of the most common cancer worldwide.
Material and methods:
The impact of phytochemicals, viability and proliferation of plant extracts on NK cells was examined in NK-92 cells using both LDH and MTT assays. The immunomodulatory properties of selected compounds were tested against human colon cancer cell line LS180 using the MTT test.
Results:
Extracts of chlorella and goji berries significantly increased NK cell proliferation, while curcumin and resveratrol did not affect this process. Curcumin, as well as extracts of chlorella and goji berries, did not impact NK viability, while resveratrol significantly increased it. LDH test revealed the cytotoxic effect of chlorella extract and curcumin in NK-92 cell cultures. On the contrary, goji berries extract significantly decreased LDH level, while resveratrol did not affect the integrity of NK cell membranes. Studies conducted in co-cultures NK cells, also directly eliminated colon cancer cells.
Conclusions:
Performed studies revealed immunomodulatory properties of goji berries extract, which improved viability and proliferation of NK cells, and above all, significantly increased their ability to recognize and eliminate colon cancer cells.
REFERENCES (63)
1.
Bishayee A, Sethi G. Bioactive natural products in cancer prevention and therapy: Progress and promise. Semin Cancer Biol. 2016; 40–41: 1–3. https://doi.org/10.1016/j.semc....
2.
Rejhová A, OpattováA, Čumová A, Slíva D, Vodička P. Natural compounds and combination therapy in colorectal cancer treatment. Eur J Med Chem. 2018; 144: 582–594. https://doi.org/10.1016/j.ejme....
3.
Kwaśnik P, Lemieszek MK, Rzeski W. Możliwości wykorzystania komórek NK w immunoterapii nowotworów. Med Og Nauk Zdr. 2020; 26(1): 8–16. https://doi.org/10.26444/monz/....
4.
Kim NH, Kim KY, Jeong HJ, Kim HM, Hong SH, Um JY. Effects of hydrolyzed Chlorella vulgaris by malted barley on the immuno-modulatory response in ICR mice and in Molt-4 cells. J Sci Food Agric. 2010; 90(9): 1551–1556. https://doi.org/10.1002/jsfa.3....
5.
Cheng D1, Wan Z, Zhang X, Li J, Li H, Wang C. Dietary Chlorella vulgaris ameliorates altered immunomodulatory functions in cyclo-phosphamide-induced immunosuppressive mice. Nutrients. 2017; 9(7): E708. https://doi.org/ 10.3390/nu9070708.
6.
Ishiguro S, Robben N, Burghart R, Cote P, Greenway S, Thakkar R, et al. Cell wall membrane fraction of Chlorella sorokiniana enhances host antitumor immunity and inhibits colon carcinoma growth in mice. Integr Cancer Ther. 2020; 19: 1534735419900555. https://doi.org/10.1177/153473....
7.
Qi J, Kim SM. Characterization and immunomodulatory activities of polysaccharides extracted from green alga Chlorella ellipsoidea. Int J Biol Macromol. 2017; 95: 106–114. https://doi.org/ 10.1016/j.ijbiomac.2016.11.039.
8.
Qi J, Kim SM. Effects of the molecular weight and protein and sulfate content of Chlorella ellipsoidea polysaccharides on their immunomodulatory activity. Int J Biol Macromol. 2018; 107(Pt A): 70–77. https://doi.org/ 10.1016/j.ijbiomac.2017.08.144.
9.
Morris HJ, Carrillo O, Almarales A, Bermudez RC, Lebequee Y, Fontaine R, et al. Immunostimulant activity of an enzymatic protein hydrolysate from green microalga Chlorella vulgaris on undernourished mice. Enzyme Microb Tech. 2007; 40: 456–460. https://doi.org/10.1016/j.enzm....
10.
Povolo C, Foschini A, Ribaudo G. Optimization of the extraction of bioactive molecules from Lycium barbarum fruits and evaluation of the antioxidant activity: a combined study. Nat Prod Res. 2019; 33(18): 2694–2698. https://doi.org/ 10.1080/14786419.2018.1460835.
11.
Lin FY, Lai YK, Yu HC, Chen NY, Chang CY, Lo HC, et al. Effects of Lycium barbarum extract on production and immunomodulatory activity of the extracellular polysaccharopeptides from submerged fermentation culture of Coriolus versicolor. Food Chem. 2008; 110(2): 446–453. https://doi.org/10.1016/j.food....
12.
Zhu PF, Zhao YL, Dai Z, Qin XJ, Yuan HL, Jin Q, et al. Phenolic amides with immunomodulatory activity from the nonpolysaccharide fraction of Lycium barbarum fruits. J Agric Food Chem. 2020; 68(10): 3079–3087. https://doi.org/ 10.1021/acs.jafc.9b07499.
13.
Gong Y, Wu J, Li ST. Immuno-enhancement effects of Lycium ruthenicum Murr. polysaccharide on cyclophosphamide-induced immuno-suppression in mice. Int J Clin Exp Med. 2015; 8(11): 20631–20637.
14.
Shakeri F, Boskabady MH. Anti-inflammatory, antioxidant, and immunomodulatory effects of curcumin in ovalbumin-sensitized rat. Biofactors. 2017; 43(4): 567–576. https://doi.org/10.1002/biof.1....
15.
Srivastava RM, Singh S, Dubey SK, Misra K, Khar A. Immunomodulatory and therapeutic activity of curcumin. Int Immunopharmacol. 2011; 11(3): 331–341. https://doi.org/10.1016/j.inti....
16.
Gao X, Kuo J, Jiang H, Deeb D, Liu Y, Divine G, et al. Immunomodulatory activity of curcumin: suppression of lymphocyte proliferation, development of cell-mediated cytotoxicity, and cytokine production in vitro. Biochem Pharmacol. 2004; 68(1): 51–61. https://doi.org/10.1016/j.bcp.....
17.
Shahid H, Shahzad M, Shabbir A, Saghir G. Immunomodulatory and anti-inflammatory potential of curcumin for the treatment of allergic asthma: effects on expression levels of pro-inflammatory cytokines and aquaporins. Inflammation. 2019; 42(6): 2037–2047. https://doi.org/ 10.1007/s10753-019-01066-2.
18.
Mohammadi A, Blesso CN, Barreto GE, Banach M, Majeed M, Sahebkar A. Macrophage plasticity, polarization and function in response to curcumin, a diet-derived polyphenol, as an immunomodulatory agent. J Nutr Biochem. 2019; 66: 1–16. https://doi.org/10.1016/j.jnut....
19.
Wang S, Li H, Zhang M, Yue LT, Wang CC, Zhang P, et al. Curcumin ameliorates experimental autoimmune myasthenia gravis by diverse immune cells. Neurosci Lett. 2016; 626: 25–34. https://doi.org/10.1016/j.neul....
20.
Fuggetta M, Mattivi F. The immunomodulating activities of resveratrol glucosides in humans. Recent Pat Food Nutr Agric. 2011; 3(2): 81–90.
21.
de Sá Coutinho D, Pacheco MT, Frozza RL, Bernardi A. Anti-Inflammatory Effects of Resveratrol: Mechanistic Insights. Int J Mol Sci. 2018; 19(6): E1812. https://doi.org/ 10.3390/ijms19061812.
22.
Euba B, López-López N, Rodríguez-Arce I, Fernández-Calvet A, Barberán M, Caturla N, et al. Resveratrol therapeutics combines both antimicrobial and immunomodulatory properties against respiratory infection by nontypeable Haemophilus influenzae. Sci Rep. 2017; 7(1): 12860. https://doi.org/ 10.1038/s41598-017-13034-7.
23.
Cui Q, Fu Q, Zhao X, Song X, Yu J, Yang Y, et al. Protective effects and immunomodulation on piglets infected with rotavirus following resveratrol supplementation. PLoS One. 2018; 13(2): e0192692. https://doi.org/10.1371/journa....
24.
Rencber SF, Ozbek SK, Eraldemir C, Sezer Z, Kum T, Ceylan S, et al. Effect of resveratrol and metformin on ovarian reserve and ultrastructure in PCOS: an experimental study. J Ovarian Res. 2018; 11(1): 55. https://doi.org/ 10.1186/s13048-018-0427-7.
25.
Kyadari M, Fatma T, Azad R, Velpandian T. Evaluation of antiangiogenic and antiproliferative potential of the organic extract of green algae Chlorella pyrenoidosa. Indian J Pharmacol. 2013; 45(6): 569–574. https://doi.org/ 10.4103/0253-7613.121366.
26.
Karakaş CY, Tekarslan Şahin H, Inan B, Özçimen D, Erginer YÖ. In vitro cytotoxic activity of microalgal extracts loaded nano-micro particles produced via electrospraying and microemulsion methods. Biotechnol Prog. 2019; 35(6): e2876. https://doi.org/ 10.1002/btpr.2876.
27.
Zhang J, Liu L, Ren Y, Chen F. Characterization of exopolysaccharides produced by microalgae with antitumor activity on human colon cancer cells. Int J Biol Macromol. 2019; 128: 761–767. https://doi.org/10.1016/j.ijbi....
28.
Kubatka P, Kapinová A, Kružliak P, Kello M, Výbohová D, Kajo K, et al. Antineoplastic effects of Chlorella pyrenoidosa in the breast cancer model. Nutrition. 2015; 31(4): 560–569. https://doi.org/ 10.1016/j.nut.2014.08.010.
29.
Gong G, Liu Q, Deng Y, Dang T, Dai W, Liu T, et al. Arabinogalactan derived from Lycium barbarum fruit inhibits cancer cell growth via cell cycle arrest and apoptosis. Int J Biol Macromol. 2020; 149: 639–650. https://doi.org/ 10.1016/j.ijbiomac.2020.01.251.
30.
Mao F, Xiao B, Jiang Z, Zhao J, Huang X, Guo J. Anticancer effect of Lycium barbarum polysaccharides on colon cancer cells involves G0/G1 phase arrest. Med Oncol. 2011; 28(1): 121–126. https://doi.org/ 10.1007/s12032-009-9415-5.
31.
Wawruszak A, Czerwonka A, Okła K, Rzeski W. Anticancer effect of ethanol Lycium barbarum (Goji berry) extract on human breast cancer T47D cell line. Nat Prod Res. 2016; 30(17): 1993–1996.
32.
Georgiev KD, Slavov IJ, Iliev IA. Antioxidant activity and anti-proliferative effects of Lycium barbarum’s (goji berry) fractions on breast cancer cell lines. Folia Med (Plovdiv). 2019; 61(1): 104–112. https://doi.org/ 10.2478/folmed-2018-0053.
33.
Chen S, Liang L, Wang Y, Diao J, Zhao C, Chen G, et al. Synergistic immuno therapeutic effects of Lycium barbarum polysaccharide and interferon-?2b on the murine Renca renal cell carcinoma cell line in vitro and in vivo. Mol Med Rep. 2015; 12(5): 6727–6737. https://doi.org/10.3892/mmr.20....
34.
Mortezaee K, Salehi E, Mirtavoos-Mahyari H, Motevaseli E, Najafi M, Farhood B, et al. Mechanisms of apoptosis modulation by curcumin: Implications for cancer therapy. J Cell Physiol. 2019; 234(8): 12537–12550. https://doi.org/ 10.1002/jcp.28122.
35.
Yue GG, Chan BC, Hon PM, Lee MY, Fung KP, Leung PC. Evaluation of in vitro anti-proliferative and immunomodulatory activities of compounds isolated from Curcuma longa. Food Chem Toxicol. 2010; 48(8–9): 2011–2020. https://doi.org/ 10.1016/j.fct.2010.04.039.
36.
Calibasi-Kocal G, Pakdemirli A, Bayrak S, Ozupek NM, Sever T, Basbinar Y, et al. Curcumin effects on cell proliferation, angiogenesis and metastasis in colorectal cancer. J BUON. 2019; 24(4): 1482–1487.
37.
Srivastava NS, Srivastava RAK. Curcumin and quercetin synergistically inhibit cancer cell proliferation in multiple cancer cells and modulate Wnt/ß-catenin signaling and apoptotic pathways in A375 cells. Phytomedicine. 2019; 52: 117–128. https://doi.org/10.1016/j.phym....
38.
Pal A, Sung B, Bhanu Prasad BA, Schuber PT Jr, Prasad S, Aggarwal BB, et al. Curcumin glucuronides: assessing the proliferative activity against human cell lines. Bioorg Med Chem. 2014; 22(1): 435–439. https://doi.org/ 10.1016/j.bmc.2013.11.006.
39.
Breuss JM, Atanasov AG, Uhrin P. Resveratrol and its effects on the vascular system. Int J Mol Sci. 2019; 20(7): 1523. https://doi.org/10.3390/ijms20....
40.
Pezzuto JM. Resveratrol: Twenty Years of Growth, Development and Controversy. Biomol Ther (Seoul). 2019; 27(1): 1–14. https://doi.org/10.4062/biomol....
41.
Carter LG, D’Orazio JA, Pearson KJ. Resveratrol and cancer: focus on in vivo evidence. Endocrine-Related Cancer 2014; 21: 209–225. https://doi.org/10.1530/ERC-13....
42.
Kim TH, Park JH, Woo JS. Resveratrol induces cell death through ROS-dependent downregulation of Notch1/PTEN/Akt signaling in ovarian cancer cells. Mol Med Rep. 2019; 19(4): 3353–3360. https://doi.org/10.3892/mmr.20....
43.
Heo JR, Kim SM, Hwang KA, Kang JH, Choi KC. Resveratrol induced reactive oxygen species and endoplasmic reticulum stress-mediated apoptosis, and cell cycle arrest in the A375SM malignant melanoma cell line. Int J Mol Med. 2018; 42(3): 1427–1435. https://doi.org/10.3892/ijmm.2....
44.
Pouyafar A, Rezabakhsh A, Rahbarghazi R, Heydarabad MZ, Shokrollahi E, Sokullu E, et al. Treatment of cancer stem cells from human colon adenocarcinoma cell line HT-29 with resveratrol and sulindac induced mesenchymal-endothelial transition rate. Cell Tissue Res. 2019; 376(3): 377–388. https://doi.org/10.1007/s00441....
45.
Liu L, Zhang Y, Zhu K, Song L, Tao M, Huang P, et al. Resveratrol inhibits glioma cell growth via targeting LRIG1. J BUON. 2018; 23(2): 403–409.
46.
Yuan L, Zhang Y, Xia J, Liu B, Zhang Q, Liu J, et al. Resveratrol induces cell cycle arrest via a p53-independent pathway in A549 cells. Mol Med Rep. 2015; 11(4): 2459–2464. https://doi.org/10.3892/mmr.20....
47.
Zadi Heydarabad M, Nikasa M, Vatanmakanian M, Azimi A, Farshdousti Hagh M. Regulatory effect of resveratrol and prednisolone on MDR1 gene expression in acute lymphoblastic leukemia cell line (CCRF-CEM): An epigenetic perspective. J Cell Biochem. 2018; 119(6): 4890–4896. https://doi.org/10.1002/jcb.26....
48.
Heiduschka G, Bigenzahn J, Brunner M, Thurnher D. Resveratrol synergistically enhances the effect of etoposide in HNSCC cell lines. Acta Otolaryngol. 2014; 134(10): 1071–1078. https://doi.org/10.3109/000164....
49.
Walle T. Bioavailability of resveratrol. Annals of the New York Academy of Sciences. Ann N Y Acad Sci. 2011; 1215: 9–15. doi: 10.1111/j.1749-6632.2010.05842.x.
50.
Koushki M, Amiri-Dashatan N, Ahmadi N, Abbaszadeh HA, Rezaei-Tavirani M. Resveratrol: A miraculous natural compound for diseases treatment. Food Sci Nutr. 2018; 6(8): 2473–2490. https://doi.org/10.1002/fsn3.8....
51.
Roncoroni L, Elli L, Dolfini E, Erba E, Dogliotti E, Terrani C, et al. Resveratrol inhibits cell growth in a human cholangiocarcinoma cell line. Liver Int. 2008; 28(10): 1426–1436. https://doi.org/ 10.1111/j.1478-3231.2008.01749.
52.
Lu CC, Chen JK. Resveratrol enhances perforin expression and NK cell cytotoxicity through NKG2D-dependent pathways. J Cell Physiol. 2010; 223(2): 343–351. https://doi.org/10.1002/jcp.22....
53.
Espinoza JL, Takami A, Trung LQ, et al. Ataxia-telangiectasia mutated kinase-mediated upregulation of NKG2D ligands on leukemia cells by resveratrol results in enhanced natural killer cell susceptibility. Cancer Sci. 2013; 104(6): 657–662. https://doi.org/10.1111/cas.12....
54.
Li T, Fan GX, Wang W, et al. Resveratrol induces apoptosis, influences IL-6 and exerts immunomodulatory effect on mouse lymphocytic leukemia both in vitro and in vivo. Int Immunopharmacol. 2007; 7(9): 1221–1231. https://doi.org/10.1016/j.inti....
55.
Segun PA, Ogbole OO, Ismail FMD, Nahar L, Evans AR, Ajaiyeoba EO, et al. Resveratrol derivatives from Commiphora africana (A. Rich.) Endl. display cytotoxicity and selectivity against several human cancer cell lines. Phytother Res. 2019; 33(1): 159–166. https://doi.org/10.1002/ptr.62....
56.
Zielińska-Przyjemska M, Kaczmarek M, Krajka-Kuźniak V, Łuczak M, Baer-Dubowska W. The effect of resveratrol, its naturally occurring derivatives and tannic acid on the induction of cell cycle arrest and apoptosis in rat C6 and human T98G glioma cell lines. Toxicol In Vitro. 2017; 43: 69–75. https://doi.org/10.1016/j.tiv.....
57.
Cao GW, Yang WG, Du P. Observation of the effects of LAK/IL-2 therapy combining with Lycium barbarum polysaccharides in the treatment of 75 cancer patients. Zhonghua Zhong Liu Za Zhi. 1994; 16(6): 428–431.
58.
Huyan T, Li Q, Yang H, et al. Protective effect of polysaccharides on simulated microgravity-induced functional inhibition of human NK cells. Carbohydr Polym. 2014; 101: 819–827. https://doi.org/10.1016/j.carb....
59.
Lee SR, Hwang HJ, Yoon JG, Bae EY, Goo KS, Cho SJ, et al. Anti-inflammatory Effect of Lycium Barbarum on Polarized Human Intestinal Epithelial Cells. Nutr Res Pract. 2019; 13(2): 95–104. https://doi.org/ 10.4162/nrp.2019.13.2.95.
60.
Juan-García A, Montesano D, Manes J, Juan C. Cytoprotective effects of carotenoids-rich extract from Lycium barbarum L. on the beauvericin-induced cytotoxicity on Caco-2 cells. Food Chem Toxicol. 2019; 133: 110798. https://doi.org/10.1016/j.fct.....
61.
Zhang M, Chen H, Huang J, Li Z, Zhu C, Zhang S. Effect of Lycium barbarum polysaccharide on human hepatoma QGY7703 cells: inhibition of proliferation and induction of apoptosis. Life Sci. 2005; 76(18): 2115–2124. https://doi.org/10.1016/j.lfs.....
62.
Miao Y, Xiao B, Jiang Z, Guo Y, Mao F, Zhao J, et al. Growth inhibition and cell-cycle arrest of human gastric cancer cells by Lycium barbarum polysaccharide. Med Oncol. 2010; 27(3): 785–790. https://doi.org/ 10.1007/s12032-009-9286-9.
63.
Suck G, Odendahl M, Nowakowska P, Seidl C, Wels WS, Klingemann HG, Tonn T. NK-92: an ‘off-the-shelf therapeutic’ for adoptive natural killer cell-based cancer immunotherapy. Cancer Immunol Immunother. 2016; 65(4): 485–892. doi: 10.1007/s00262-015-1761-x.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.