Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
RESEARCH PAPER
Cathelicidin influence on pathological activation of Wnt pathway in murine model of hypersensitivity pneumonitis
1
Department of Medical Biology, Institute of Rural Health, Lublin, Poland
2
Digital Global Health Working Group, Institute of Global Health, University Hospital, Heidelberg, Germany
3
Department of Biological Health Hazards and Parasitology, Institute of Rural Health, Lublin, Poland
4
Department of Pneumonology, Oncology and Allergology, Medical University, Lublin, Poland
Corresponding author
Marta Kinga Lemieszek
Department of Medical Biology, Institute of Rural Health, Lublin, Poland, Poland
Department of Medical Biology, Institute of Rural Health, Lublin, Poland, Poland
Ann Agric Environ Med. 2022;29(3):358-364
KEYWORDS
extrinsic allergic alveolitisPantoea agglomeranslung fibrosisdefence peptidesimmune peptidesWnt/ β-Catenin pathway
TOPICS
ABSTRACT
Introduction and objective:
Cathelicidin (CRAMP) is a defence peptide with a wide range of biological responses including antimicrobial, immunomodulatory and wound healing. Furthermore, our previous studies suggested the possibility of using cathelicidin in the prevention and treatment of pulmonary fibrosis in the course of hypersensitivity pneumonitis (HP). The molecular mechanism of CRAMP action, however, was not fully explained. Due to the fact that several studies indicated the Wnt signals pathways as a key player in wound healing and fibrosis, studies focused on this pathway in order to explain the above-mentioned therapeutic potential of cathelicidin in HP development.
Material and methods:
The study was conducted in a murine model of HP, wherein lung fibrosis is induced in mice strain C57BL/6J by chronic exposure to saline extract of P. agglomerans (SE-PA). Cathelicidin was administered in the an form of aerosol during HP development. Changes in the expression of genes and proteins involved in signals transduction in the Wnt/β-Catenin pathway were examined in lung tissue homogenates by RealTime PCR and Western Blotting, respectively.
Results:
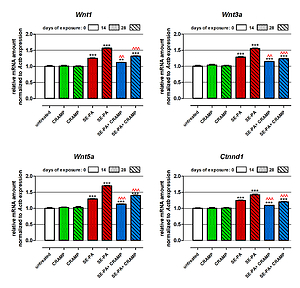
The study revealed that cathelicidin decreased the elevated level of components of the Wnt/β-Catenin pathway (Ctnnd1/β-Catenin, Wnt1/WNt1, Wnt3a/Wnt3a, Wnt5a/Wnt5a) in the murine model of HP. Furthermore, CRAMP administered together with SE-PA inhibited the transcription function of β-catenin, leading to a decrease in abnormal expression of profibrotic molecules: Cyclin D1, c-Myc, MMP-7. Nevertheless, cathelicidin was not able to completely neutralize the negative changes induced by SE-PA.
Conclusions:
The study demonstrated the beneficial effect of exogenous cathelicidin on signals transduction in the Wnt/β-Catenin pathway, which may prevent fibrosis development in HP.
Cathelicidin (CRAMP) is a defence peptide with a wide range of biological responses including antimicrobial, immunomodulatory and wound healing. Furthermore, our previous studies suggested the possibility of using cathelicidin in the prevention and treatment of pulmonary fibrosis in the course of hypersensitivity pneumonitis (HP). The molecular mechanism of CRAMP action, however, was not fully explained. Due to the fact that several studies indicated the Wnt signals pathways as a key player in wound healing and fibrosis, studies focused on this pathway in order to explain the above-mentioned therapeutic potential of cathelicidin in HP development.
Material and methods:
The study was conducted in a murine model of HP, wherein lung fibrosis is induced in mice strain C57BL/6J by chronic exposure to saline extract of P. agglomerans (SE-PA). Cathelicidin was administered in the an form of aerosol during HP development. Changes in the expression of genes and proteins involved in signals transduction in the Wnt/β-Catenin pathway were examined in lung tissue homogenates by RealTime PCR and Western Blotting, respectively.
Results:
The study revealed that cathelicidin decreased the elevated level of components of the Wnt/β-Catenin pathway (Ctnnd1/β-Catenin, Wnt1/WNt1, Wnt3a/Wnt3a, Wnt5a/Wnt5a) in the murine model of HP. Furthermore, CRAMP administered together with SE-PA inhibited the transcription function of β-catenin, leading to a decrease in abnormal expression of profibrotic molecules: Cyclin D1, c-Myc, MMP-7. Nevertheless, cathelicidin was not able to completely neutralize the negative changes induced by SE-PA.
Conclusions:
The study demonstrated the beneficial effect of exogenous cathelicidin on signals transduction in the Wnt/β-Catenin pathway, which may prevent fibrosis development in HP.
ACKNOWLEDGEMENTS
The studies were supported by the National Science Centre, Poland [Grant No, 2015/19/D/NZ7/02952, 2016].
REFERENCES (35)
1.
Ziesche R, Golec M, Samahaet E. The RESOLVE concept: approaching pathophysiology of fibroproliferative disease in aged individuals. Biogerontol. 2013;14(6):679–685. https://doi.org/10.1007/s10522....
2.
Spagnolo P, Rossi G, Cavazza A, et al. Hypersensitivity pneumonitis: a comprehensive review. J Investig Allergol Clin Immunol. 2015;25(4): 237–250.
3.
Nogueira R, Melo N, Novais e Bastos H, et al. Hypersensitivity pneumonitis: Antigen diversity and disease implications. Pulmonology. 2019;25(2):97–108. https://doi.org/10.1016/j.pulm....
4.
Dutkiewicz J, Mackiewicz B, Lemieszek MK, et al. Pantoea agglomerans: a mysterious bacterium of evil and good. Part I. Deleterious effects: Dust-borne endotoxins and allergens – focus on cotton dust. Ann Agric Environ Med. 2015;22(4):576–588. https://doi.org/10.5604/123219....
5.
Dutkiewicz J, Mackiewicz B, Lemieszek MK, et al. Pantoea agglomerans: a mysterious bacterium of evil and good. Part II – Deleterious effects: Dustborne endotoxins and allergens – focus on grain dust, other agricultural dusts and wood dust. Ann Agric Environ Med. 2016;23(1):6–29. https://doi.org/10.5604/123219....
6.
Hyldgaard C, Hilberg O, Muller A, et al. A cohort study of interstitial lung diseases in central Denmark. Respir Med. 2014;108:793–799. https://doi.org/10.1016/j.rmed....
7.
Rittig AH, Hilberg O, Ibsen R, et al. Incidence, comorbidity and survival rate of hypersensitivity pneumonitis: a national population-based study. ERJ Open Res. 2019;5(4):00259–2018. https://doi.org/10.1183/231205....
8.
Golec M, Skórska C, Lemieszek M, et al. A novel inhalation challenge set to study animal model of allergic alveolitis. Ann Agric Environ Med. 2009:16:173–175.
9.
Lemieszek M, Chilosi M, Golec M, et al. Mouse model of hypersensitivity pneumonitis after inhalation exposure to different microbial antigens associated with organic dusts. Ann Agric Environ Med. 2011;18(1):71–80.
10.
Lemieszek MK, Chilosi M, Golec M, et al. Age influence on hypersensitivity pneumonitis induced in mice by exposure to Pantoea agglomerans. Inhal Toxicol. 2013;25(11):640–650. https://doi.org/10.3109/089583....
11.
Lemieszek MK, Sawa-Wejksza K, Golec M, et al. Beneficial impact of cathelicidin on hypersensitivity pneumonitis treatment – In vivo studies. Plos One. 2021;16(5):e0251237. https://doi.org/10.1371/journa....
12.
Sakai N, Tager AM. Fibrosis of two: epithelial cell-fibroblast interactions in pulmonary fibrosis. Biochim Biophys Acta. 2013;1832(7):911–921. https://doi.org/10.1016/j.bbad....
13.
Vaughan AE, Chapman HA. Regenerative activity of the lung after epithelial injury. Biochim Biophys Acta. 2013;1832(7):922–930. https://doi.org/10.1016/j.bbad....
14.
Rockey DC, Bell PD, Hil JA. Fibrosis–a common pathway to organ injury and failure. N Engl J Med. 2015;372(12):1138–1149. https://doi.org/10.1056/nejmra....
15.
Shaykhiev R, Beisswenger C, Kändler K, et al. Human endogenous antibiotic LL-37 stimulates airway epithelial cell proliferation and wound closure. Am J Physiol Lung Cell Mol Physiol. 2005;289(5):L842–848. https://doi.org/10.1152/ajplun....
16.
Alford MA, Baquir B, Santana FL, et al. Cathelicidin host defense peptides and inflammatory signaling: striking a balance. Front Microbiol. 2020;11:1902. https://doi.org/10.3389/fmicb.....
17.
Golec M, Reichel C, Lemieszek M, et al. Cathelicidin LL-37 in bronchoalveolar lavage and epithelial lining fluids from COPD patients and healthy individuals. J Biol Regul Homeost Agents. 2012:26(4):617–625.
18.
Golec M, Reichel C, Lemieszek M, et al. Cathelicidin LL-37 in bronchoalveolar lavage and epithelial lining fluids from healthy individuals and sarcoidosis patients. J Biol Regul Homeost Agents. 2014:28(1):73–79.
19.
Golec M, Reichel C, Mackiewicz B, et al. Cathelicidin LL-37, granzymes, TGF-beta1 and cytokines levels in induced sputum from farmers with and without COPD. Ann Agric Environ Med. 2009:16:289–297.
20.
Burgy O, Königshoff M. The WNT signaling pathways in wound healing and fibrosis. Matrix Biol. 2018;68–69:67–80. https://doi.org/10.1016/j.matb....
21.
Dutkiewicz J. Exposure to dust-borne bacteria in agriculture. I. Environmental studies. Arch Environ Health. 1978a;33:250–259.
22.
Dutkiewicz J. Exposure to dust-borne bacteria in agriculture. II. Immunological survey. Arch Environ Health. 1978b;33:260–270.
23.
Komiya Y, Habas R. Wnt signal transduction pathways. Organogenesis. 2008;4(2):68–75. https://doi.org/10.4161/org.4.....
24.
Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. https://doi.org/10.1146/annure....
25.
Guo Y, Xiao L, Sun L, et al. Wnt/beta-Catenin signaling: a promising new target for fibrosis diseases. Physiol Res. 2012;61(4):337–346. https://doi.org/10.33549/physi....
26.
Dees C, Distler JH. Canonical Wnt signalling as a key regulator of fibrogenesis – implications for targeted therapies? Exp Dermatol. 2013;22(11):710–713. https://doi.org/10.1111/exd.12....
27.
Hu HH, Cao G, Wu XQ, et al. Wnt signaling pathway in aging-related tissue fibrosis and therapies. Ageing Res Rev. 2020;60:101063. https://doi.org/10.1016/j.arr.....
28.
Chilosi M, Poletti V, Zamo A, et al. Aberrant Wnt/beta-Catenin pathway activation in idiopathic pulmonary fibrosis. Am J Pathol. 2003;162:1497–1502. https://doi.org/10.1016/s0002-....
29.
Konigshoff M, Balsara N, Pfaff EM, et al. Functional Wnt signaling is increased in idiopathic pulmonary fibrosis. PLoS One. 2008;3(5):e2142. https://doi.org/10.1371/journa....
30.
Lam AP, Flozak AS, Russell S, et al. Nuclear beta-Catenin is increased in systemic sclerosis pulmonary fibrosis and promotes lung fibroblast migration and proliferation. Am J Respir Cell Mol Biol. 2011;45(5):915–922. https://doi.org/10.1165/rcmb.2....
31.
Akhmetshina A, Palumbo K, Dees C, et al. Activation of canonical Wnt signalling is required for TGF-beta-mediated fibrosis, Nat Commun. 2012;3:735. https://doi.org/10.1038/ncomms....
32.
Zuo F, Kaminski N, Eugui E, et al. Gene expression analysis reveals matrilysin as a key regulator of pulmonary fibrosis in mice and humans. Proc Natl Acad Sci U S A. 2002;99:6292–6297. https://doi.org/10.1073/pnas.0....
33.
Disayabutr EK, Kim SI, Cha G, et al. miR-34 miRNAs regulate cellular senescence in type II alveolar epithelial cells of patients with idiopathic pulmonary fibrosis. PLoS One. 2016;11(6):e0158367. https://doi.org/10.1371/journa....
34.
Qin H, Tang Y, Mao Y, et al. C-MYC induces idiopathic pulmonary fibrosis via modulation of miR-9–5p-mediated TBPL1. Cell Signal. 2022;93:110274. https://doi.org/10.1016/j.cell....
35.
Landi C, Bergantini L, Cameli P, et al. Idiopathic pulmonary fibrosis serum proteomic analysis before and after nintedanib therapy. Sci Rep. 2020;10(1):9378. https://doi.org/10.1038/s41598....
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.