Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
RESEARCH PAPER
Modification of exhaled air nitric oxide in patients with asthma – cortisone monotherapy or dual inhalation therapy?
1
Iuliu Hatieganu’ University of Medicine and Pharmacy, Cluj-Napoca, Romania
2
Department of Pneumology, ’Iuliu Hatieganu’ University of Medicine and Pharmacy, Cluj-Napoca, Romania
3
Department of Internal Medicine, ‚Iuliu Hatieganu’ University of Medicine and Pharmacy, Cluj-Napoca, Romania
4
MD student ‚Iuliu Hatieganu’ University of Medicine and Pharmacy, Cluj-Napoca, Romania
5
Faculty of Veterinary Medicine, University of Agricultural Science and Veterinary Medicine, Cluj-Napoca, Romania
6
Department of Rehabilitation, ‘Iuliu Hatieganu’ University of Medicine and Pharmacy Cluj-Napoca, Cluj-Napoca, Romania
Corresponding author
Rodica Ana Ungur
, Department of Rehabilitation, ‚Iuliu Hatieganu’ University of Medicine and Pharmacy Cluj-Napoca, 46-50 Viilor Street, 400347, Cluj-Napoca, Romania.
, Department of Rehabilitation, ‚Iuliu Hatieganu’ University of Medicine and Pharmacy Cluj-Napoca, 46-50 Viilor Street, 400347, Cluj-Napoca, Romania.
Ann Agric Environ Med. 2021;28(1):89-93
KEYWORDS
TOPICS
ABSTRACT
Introduction:
Asthma, a chronic lung disease, is a major health challenge worldwide with increased addressability to health services. There are different asthma phenotypes, which have different evolution and can be specifically tracked. The measurement of fractional expired nitric oxide (FeNo) with different devices reflects the eosinophilic inflammation of the airways, and can be used to evaluate the allergic phenotype and predict the treatment responses. The new GINA (Global Initiative for Asthma) guideline recommends FeNO monitoring to assess adherence to cortisone treatment in high doses before prescribing biological treatment, and as a means of monitoring the decrease in oral corticosteroid treatment.
Objective:
The aim of the study is to analyze the applicability of FeNO in monitoring response to therapy.
Material and methods:
An observational study was carried out on 129 subjects with a previously established diagnosis of asthma. The research was based on the determination of FeNO with NObreath. Those with intermediate FeNO received a low dose of inhaled corticosteroids in mono/dual therapy, those with increased FeNO received medium ICS mono/dual therapy. FeNO testing, its values and doses of ICS were below the the ATS / ERS guidelines.
Results:
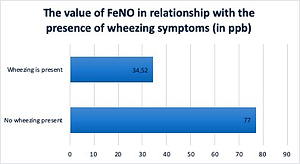
FeNO reduction is strictly dependent on the cortisone dose. Applying the dual therapy from the beginning does not bring additional benefits in comparison with cortisone in monotherapy, in terms of FeNO value.
Conclusions:
Recommendations that include FeNO testing can help monitor response to treatment.
Asthma, a chronic lung disease, is a major health challenge worldwide with increased addressability to health services. There are different asthma phenotypes, which have different evolution and can be specifically tracked. The measurement of fractional expired nitric oxide (FeNo) with different devices reflects the eosinophilic inflammation of the airways, and can be used to evaluate the allergic phenotype and predict the treatment responses. The new GINA (Global Initiative for Asthma) guideline recommends FeNO monitoring to assess adherence to cortisone treatment in high doses before prescribing biological treatment, and as a means of monitoring the decrease in oral corticosteroid treatment.
Objective:
The aim of the study is to analyze the applicability of FeNO in monitoring response to therapy.
Material and methods:
An observational study was carried out on 129 subjects with a previously established diagnosis of asthma. The research was based on the determination of FeNO with NObreath. Those with intermediate FeNO received a low dose of inhaled corticosteroids in mono/dual therapy, those with increased FeNO received medium ICS mono/dual therapy. FeNO testing, its values and doses of ICS were below the the ATS / ERS guidelines.
Results:
FeNO reduction is strictly dependent on the cortisone dose. Applying the dual therapy from the beginning does not bring additional benefits in comparison with cortisone in monotherapy, in terms of FeNO value.
Conclusions:
Recommendations that include FeNO testing can help monitor response to treatment.
ACKNOWLEDGEMENTS
The first two authors have equal rights to this paper.
Ruta VM, Motoc NS, Todea DA, Alexescu TG, Valean D, Cozac S, Coste SC, Codea RA, Ungur RA, Pop CM, Milena AM. Modification of exhaled air nitric oxide in patients with asthma: cortisone monotherapy or dual inhalation therapy? Ann Agric Environ Med.doi: 10.26444/aaem/130712
REFERENCES (40)
1.
Global Strategy for Asthma Management and Prevention (2018 update) https://ginasthma.org/wp-conte... (access:2020.09.08).
2.
Masefield S, Edwards J, Hansen K, Hamerlijnck D, Lisspers K, Schee M van der, et al. The future of asthma research and development: a roadmap from the European Asthma Research and Innovation Partnership (EARIP). Eur Respir J. 2017; 49(5).
3.
Croitoru A, Bogdan MA. [Evidences related to pulmonary rehabilitation in the respiratory pathology]. Pneumol Buchar Rom. 2014 Jun; 63(2): 88–90, 92–5.
4.
Nistor AR, Onac I, Stefanescu A, Borda IM, Ciortea V, Ungur R. EBSCOhost | 108800415 | The role of singing therapy in pulmonary rehabilitation. Palestrica of the Third Millennium Civilization & Sport. 2015; 16(2).
5.
Georas SN, van Wijngaarden E, Rich DQ. Air pollution and asthma incidence: doubt no more? Lancet Respir Med. 2015 Dec; 3(12): 902–3.
6.
Rezaei Vandchali N, Koolivand A, Ranjbar A, et al. Oxidative toxic stress and p53 level in healthy subjects occupationally exposed to outdoor air Pollution – a cross-sectional study in Iran. Ann Agric Environ Med. 2020. doi: 10.26444/aaem/126313.
7.
Ungur R, Dronca M, Crăciun EC, Rusu RL, Văleanu M, Onac I, et al. Improvement of total antioxidant status, a possible bioeffect of the ultrasound therapy – a pilot study. Rev Romana Med Lab. 2011; 19(2): 177–83.
8.
Pignatelli P, Menichelli D, Pastori D, Violi F. Oxidative stress and cardiovascular disease: new insights. Kardiol Pol. 2018; 76(4): 713–22.
9.
Cozma A, Sitar-Taut A, Orăşan O, Leucuta D, Alexescu T, Stan A, et al. Determining Factors of Arterial Stiffness in Subjects with Metabolic Syndrome. Metab Syndr Relat Disord. 2018 Sep 5; 16(9): 490–6.
10.
Barnes PJ, Liew FY. Nitric oxide and asthmatic inflammation. Immunol Today. 1995 Mar; 16(3): 128–30.
11.
Lemière C, Ernst P, Olivenstein R, Yamauchi Y, Govindaraju K, Ludwig MS, et al. Airway inflammation assessed by invasive and noninvasive means in severe asthma: Eosinophilic and noneosinophilic phenotypes. J Allergy Clin Immunol. 2006 Nov 1; 118(5): 1033–9.
12.
Matacuta I, Neamtu MB, Bodrug N, Dobrota L, Neamtu ML, Caprita H. Metode de explorare funcţională pulmonară. Rev Stiintifico-Pract Info-Med. 2013; 1(21): 32–6.
13.
Hoyte FCL, Gross LM, Katial RK. Exhaled Nitric Oxide: An Update. Immunol Allergy Clin North Am. 2018; 38(4): 573–85.
14.
Pocket guide for asthma management and prevention (GINA-2019) https://ginasthma.org/wp-conte... (access:2020.09.08).
15.
Dweik RA, Boggs PB, Erzurum SC, Irvin CG, Leigh MW, Lundberg JO, et al. An Official ATS Clinical Practice Guideline: Interpretation of Exhaled Nitric Oxide Levels (FeNO) for Clinical Applications. Am J Respir Crit Care Med. 2011 Sep 1; 184(5): 602–15.
16.
Terada A, Fujisawa T, Togashi K, Miyazaki T, Katsumata H, Atsuta J, et al. Exhaled Nitric Oxide Decreases during Exercise-induced Bronchoconstriction in Children with Asthma. Am J Respir Crit Care Med. 2001 Nov 15; 164(10): 1879–84.
18.
Malinovschi A, Backer V, Harving H, Porsbjerg C. The value of exhaled nitric oxide to identify asthma in smoking patients with asthma-like symptoms. Respir Med. 2012 Jun 1; 106(6): 794–801.
19.
Bjermer L, Alving K, Diamant Z, Magnussen H, Pavord I, Piacentini G, et al. Current evidence and future research needs for FeNO measurement in respiratory diseases. Respir Med. 2014 Jun 1; 108(6): 830–41.
20.
Trofor A, Petris O, Trofor L, Man MA, Filipeanu D, Miron R. Biochemistry in Assessing Tobacco Exposure – Smokers versus Non-smokers Correlations with clinical practice. Rev Chim. 2017 Jun 15; 68(5): 1002–6.
21.
Trofor AC, Man MA, Marginean C, Dumitru F, Trofor L. Smoking cessation for free: outcomes of a study of three Romanian clinics. Open Med. 2016 Dec 30; 11(1): 605–10.
22.
Man MA, Man SC, Motoc NŞ, Pop CM, Trofor AC. Fatal hypersensitivity pneumonitis after chemical occupational exposure. Romanian J Morphol Embryol Rev Roum Morphol Embryol. 2017; 58(2): 627–34.
23.
Sastre J, Costa C, del Garcia Potro M, Aguado E, Mahillo I, Fernández-Nieto M. Changes in exhaled nitric oxide after inhalation challenge with occupational agents. J Investig Allergol Clin Immunol. 2013; 2 3 (6): 4 21–7.
24.
Rhee H, Love T, Mammen J. Comparing Asthma Control Questionnaire (ACQ) and National Asthma Education and Prevention Program (NAEPP) asthma control criteria. Ann Allergy Asthma Immunol. 2019 Jan 1; 122(1): 58–64.
26.
Shim JY. Association of wheezing phenotypes with fractional exhaled nitric oxide in children. Korean J Pediatr. 2014 May; 57(5): 211–6.
27.
LaForce C, Brooks E, Herje N, Dorinsky P, Rickard K. Impact of exhaled nitric oxide measurements on treatment decisions in an asthma specialty clinic. Ann Allergy Asthma Immunol Off Publ Am Coll Allergy Asthma Immunol. 2014 Dec; 113(6): 619–23.
28.
Tudorache E, Fildan AP, Frandes M, Dantes E, Tofolean DE. Aging and extrapulmonary effects of chronic obstructive pulmonary disease. Clin Interv Aging. 2017 Aug 16; 12: 1281–7.
29.
Bateman ED, Hurd SS, Barnes PJ, Bousquet J, Drazen JM, FitzGerald JM, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008; 31(1): 143–78.
30.
Hauber H-P, Gotfried M, Newman K, Danda R, Servi RJ, Christodoulopoulos P, et al. Effect of HFA-flunisolide on peripheral lung inflammation in asthma. J Allergy Clin Immunol. 2003 Jul; 112(1): 58–63.
31.
Hossny E, Rosario N, Lee BW, Singh M, El-Ghoneimy D, SOH JY, et al. The use of inhaled corticosteroids in pediatric asthma: update. World Allergy Organ J https://www.ncbi.nlm.nih.gov/p... (access: 2020.09.08).
32.
Wang Z, Pianosi P, Keogh K, Zaiem F, Alsawas M, Alahdab F, et al. The Clinical Utility of Fractional Exhaled Nitric Oxide (FeNO) in Asthma Management. Agency for Healthcare Research and Quality; https://effectivehealthcare.ah... (access: 2020.09.08).
33.
Zetterström O, Buhl R, Mellem H, Perpiñá M, Hedman J, O’Neill S, et al. Improved asthma control with budesonide/formoterol in a single inhaler, compared with budesonide alone. Eur Respir J. 2001 Aug 1; 18(2): 262–8.
34.
Greening AP, Ind PW, Northfield M, Shaw G. Added salmeterol versus higher-dose corticosteroid in asthma patients with symptoms on existing inhaled corticosteroid. The Lancet. 1994 Jul 23; 344(8917): 219–24.
35.
Fingleton J, Hardy J, Baggott C, Pilcher J, Corin A, Hancox RJ, et al. Description of the protocol for the PRACTICAL study: a randomised controlled trial of the efficacy and safety of ICS/LABA reliever therapy in asthma. BMJ Open Respir Res https://www.ncbi.nlm.nih.gov/p... (access:2020.09.08).
36.
Budin CE, Maierean AD, Ianosi ES, Socaci A, Buzoianu AD, Alexescu TG, et al. Nocturnal Hypoxemia, a Key Parameter in Overlap Syndrome. Rev Chim. 2019 Mar 15; 70(2): 449–54.
37.
Liu L, Urban P, Hunt JF, Wilkinson P, Laning K, Gaston B. Changes in Exhaled Nitric Oxide and Breath pH during Fluticasone Wean in Asthma. Respiration. 2010; 79(3): 193–9.
38.
Malinovschi A, Van Muylem A, Michiels S, Michils A. FeNO as a predictor of asthma control improvement after starting inhaled steroid treatment. Nitric Oxide. 2014; 40: 110–6.
39.
Jatakanon A, Uasuf C, Maziak W, Lim S, Chung KF, Barnes PJ. Neutrophilic Inflammation in Severe Persistent Asthma. Am J Respir Crit Care Med. 1999 Nov 1; 160(5): 1532–9.
40.
Sl J, P H, Jo C, Em F, Rj H, Cr M, et al. Exhaled NO and assessment of anti-inflammatory effects of inhaled steroid: dose-response relationship. Eur Respir J. 2002 Sep 1; 20(3): 601–8.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.