Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
RESEARCH PAPER
Pilot research on gastrointestinal parasites of the Tatra chamois (Rupicapra rupicapra tatrica)
1
Institute of Parasitology of the Slovak Academy of Sciences, Slovak Republic
2
TANAP administration in Tatranská Lomnica, Slovak Republic
3
University of Veterinary Medicine and Pharmacy in Košice, Slovak Republic
4
Tatra National Park, Poland
Corresponding author
Zuzana Hurníková
Institute of Parasitology of the Slovak Academy of Sciences, Hlinkova 3, 04001, Košice, Slovak Republic
Institute of Parasitology of the Slovak Academy of Sciences, Hlinkova 3, 04001, Košice, Slovak Republic
Ann Agric Environ Med. 2022;29(4):513-517
KEYWORDS
TOPICS
ABSTRACT
Introduction and objective:
The Tatra chamois (Rupicapra rupicapra tatrica) is a significant representative of the High Tatra Mountains endemic fauna species. In terms of health hazards for these animals, an important role is played by parasitic infections that can lead to a significant depletion of the entire population.
Objective:
The aim of the study was to describe the occurrence of gastrointestinal parasites of Tatra chamois in the current environmental and climatic conditions of the High Tatra Mountains.
Material and methods:
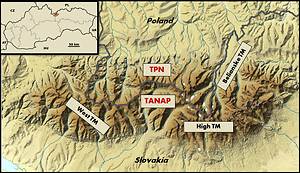
During the pilot project in 2014 – 2017, a total of 494 chamois faecal samples were collected from the Slovak High Tatra and 114 samples in the Polish part of the mountain and examined using standard coprological methods.
Results:
The results revealed that the overall positivity for gastrointestinal parasites in chamois of the Slovak High Tatra reached 74.7%. Most frequent were protozoa – Eimeria spp. (42.7%), helminths were represented by Moniezia spp. tapeworms (23.5%), eggs of GIS-strongylids (7.1%), and sporadically Capillaria spp. (1.4%). The chamois from the Polish Tatra Mts. were infected with Eimeria spp. (43.9%), GIT-strongylids (9.6%), and Moniezia spp. (6.1%). Parasitic infection was determined in 59.6 % of faecal samples from the Polish part of the mountains. Statistical analyses revealed a significant difference in Moniezia spp. occurrence in different Slovak Tatra Mts. Regions, as well as between Slovak and Polish Tatra Mts.
Conclusions:
Initial research on the gastrointestinal parasites of the Tatra chamois revealed one indisputable finding – a relatively high prevalence of the genus Moniezia, which is closely linked to the climate and microclimate conditions of the mountains. Further intensive research on parasite composition and distribution in Tatra chamois is needed in broader temporal, ecological, and zoological contexts.
The Tatra chamois (Rupicapra rupicapra tatrica) is a significant representative of the High Tatra Mountains endemic fauna species. In terms of health hazards for these animals, an important role is played by parasitic infections that can lead to a significant depletion of the entire population.
Objective:
The aim of the study was to describe the occurrence of gastrointestinal parasites of Tatra chamois in the current environmental and climatic conditions of the High Tatra Mountains.
Material and methods:
During the pilot project in 2014 – 2017, a total of 494 chamois faecal samples were collected from the Slovak High Tatra and 114 samples in the Polish part of the mountain and examined using standard coprological methods.
Results:
The results revealed that the overall positivity for gastrointestinal parasites in chamois of the Slovak High Tatra reached 74.7%. Most frequent were protozoa – Eimeria spp. (42.7%), helminths were represented by Moniezia spp. tapeworms (23.5%), eggs of GIS-strongylids (7.1%), and sporadically Capillaria spp. (1.4%). The chamois from the Polish Tatra Mts. were infected with Eimeria spp. (43.9%), GIT-strongylids (9.6%), and Moniezia spp. (6.1%). Parasitic infection was determined in 59.6 % of faecal samples from the Polish part of the mountains. Statistical analyses revealed a significant difference in Moniezia spp. occurrence in different Slovak Tatra Mts. Regions, as well as between Slovak and Polish Tatra Mts.
Conclusions:
Initial research on the gastrointestinal parasites of the Tatra chamois revealed one indisputable finding – a relatively high prevalence of the genus Moniezia, which is closely linked to the climate and microclimate conditions of the mountains. Further intensive research on parasite composition and distribution in Tatra chamois is needed in broader temporal, ecological, and zoological contexts.
REFERENCES (24)
1.
Urban P, Malina R. Reintroduction of Tatra chamois (Rupicapra rupicapra tatrica) in the Low Tatras Mts. (Central Slovakia). Quaestiones rerum naturalium 2017; 4(1): 51–134.
2.
The IUCN Red List of Threatened Species. Version 2017–3. https://www.iucnredlist.org/se... (access: 2021.6.28).
3.
Rossi L, Tizzani P, Rambozzi L, et al. Sanitary Emergencies at the Wild/Domestic Caprines Interface in Europe. Animals 2019; 9:922. https://www.mdpi.com/2076-2615....
4.
Vengušt G, Kuhar U, Jerina K, et al. Passive Disease Surveillance of Alpine Chamois (Rupicapra r. rupicapra) in Slovenia between 2000 and 2020. Animals 2022; 12: 1119. https://doi.org/10.3390/ani120....
5.
Mituch J, Hovorka J, Hovorka I, et al. Helminths and helmintocenoses of wild game in the ecological conditions of the Carpathian Arc (in Slovak). Final report, Helminthological Institute SAV Košice; 1984. pp. 81.
6.
Štefančíková A, Chovancová B, Hájek B, et al. Revision of chamois infection by lung nematodes under ecological conditions of national parks of Slovakia with respect to ongoing global climate changes. Helminthologia. 2011;48:145–154. https://doi.org/10.2478/s11687....
7.
Vido J, Tadesse T, Šustek Z, et al. Drought Occurrence in Central European Mountainous region (Tatra National Park, Slovakia) within the period 1961–2010. Adv Meteorol. 2016; Art. ID 248728. https://doi.org/10.1155/2015/2....
8.
Hlôška L, Chovancová B, Chovancová G, et al. Influence of climatic factors on the population dymnamics of small mammals (Rodentia, Soricomorpha) on the sites affected by windstorm in the High Tatra Mts. Folia Oecologica. 2016; 43(1):12–20.
9.
Reiczigel J, Marozzi M, Fábián I, et al. Biostatistics for Parasitologists – A Primer to Quantitative Parasitology. Trends Parasitol. 2019; 35(4): 277–281. https://doi.org/10.1016/j.pt.2....
10.
Gortázar Ch. Stepping Up from Wildlife Disease Surveillance to Integrated Monitoring of Shared Infections: The Role of Hunters. European Wildlife Disease Association Network Meeting, 2021 Aug 30, https://ewda.org/wp-content/up... (access: 2022.8.8).
11.
Kanchev K. Helminthological status of Balkan chamois from Bulgarian Rhodope Mountains. Tradit Mod Vet Med. 2020;1(10):34–39.
12.
Ribarić P, Bujanić M, Šprem N, et al. Gastrointestinal parasites of Alpine chamois (Rupicapra rupicapra rupicapra): coprology vs. intestine examination. Abstracts of the III International Rupicapra Symposium, 2021 June 16–18; Croatia. University of Zagreb, Faculty of Agriculture, 2021.
13.
Morrondo P, Vázquez L, Diez-Baňos P. Parasitic infections of wild ruminants in Spain with special attention to roe deer and chamois. Parassitologia. 2010;52(1–2):155–158.
14.
Martínez-Guijosa J, Martínez-Carrasco C, López-Oivera JR, et al. Male-biased gastrointestinal parasitism in a nearly monomorphic mountain ungulate. Parasit Vectors. 2015;8:165. https://parasitesandvectors.bi....
15.
Taylor MA, Coop RP, Wall EO. Veterinary parasitology, Fourth Edition, Wiley Blackwell, UK; 2016. p. 336–342.
16.
Zemanová B, Hájková P, Hájek B, et al. Extremely low genetic variation in endangered Tatra chamois and evidence for hybridization with an introduced Alpine population. Conserv Genet 2015; 16(3):729–741. https://link.springer.com/arti....
17.
Ciach M, Peksa L. Human-induced environmental changes influence habitat use by an ungulate over the long term. Curr Zool. 2019; 65(2):129–137. https://doi.org/10.1093/cz/zoy....
18.
Hoby S, Schwarzenberger F, Doherr MG, et al. Steroid hormone realted male biased parasitism in chamois, Rupicapra rupipcapra rupicapra. Vet Parasitol 2006;138:337–348. https://doi.org/10.1016/j.vetp....
19.
Babják M, Königová A, Urda-Dolinská M, et al. Gastrointestinal helminth infections of dairy goats in Slovakia. Helminthologia. 2017; 54, 3: 211 – 217. https://doi.org/10.1515/helm-2....
20.
Miko L. Damaeus lupus n.sp. (Acarina), a new oribatid mite species from the Carpathian wilderness (Tatra Mountains, Slovakia). Acarologia 2016; 56(3):279–292. https://doi.org/10.1051/acarol....
21.
Václav R, Kalúz S. The effect of herbivore faeces in the edaphic mite community: implications for tapeworm transmission. Exp Appl Acarol. 2014; 62:377–390. https://link.springer.com/arti....
22.
Reena KK, Jobin T, Nirmal Ch, et al. Examination of oribatid mites for cysticercoids of Moniezia sp. Int J Vet Sci Res. 2015; 1(2):31–35. https://doi.org/10.18488/journ....
23.
Akrami MA, Mostowfizadeh-Ghalamfarsa R, Ebrahimi F, et al. Molecular detection of Moniezia spp. (Cestoda) in Pergalumna persica (Acari: Oribatida) in Iran. Syst Appl Acarol. 2018; 23(10):1931–1939. https://doi.org/10.11158/saa.2....
24.
Roczeń-Karczmarz M, Tomczuk K. Oribatid mites as vectors of invasive diseases. Acarologia. 2016; 56(4): 613–623. https://doi.org/10.1051/acarol....
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.