Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
RESEARCH PAPER
Insulin receptors in the CA1 field of hippocampus and selected blood parameters in diabetic rats fed with bilberry fruit
1
Faculty of Veterinary Medicine, Department of Animal Anatomy and Histology, University of Life Sciences, Lublin, Poland
2
Faculty of Food Science and Biotechnology, Department of Biotechnology, Microbiology and Human Nutrition, University of Life Sciences, Lublin, Poland
Corresponding author
Kamila Borowiec
University of Life Sciences, Faculty of Food Science and Biotechnology, Department of Biotechnology, Microbiology and Human Nutrition, Skromna 8, 20-704, Lublin, Poland
University of Life Sciences, Faculty of Food Science and Biotechnology, Department of Biotechnology, Microbiology and Human Nutrition, Skromna 8, 20-704, Lublin, Poland
Ann Agric Environ Med. 2021;28(3):430-436
KEYWORDS
TOPICS
ABSTRACT
Introduction:
Bilberry fruit is believed to be a promising factor in the treatment of diabetes mellitus. Chronic hyperglycaemia affects the function of the central nervous system, which may be manifested as changes in hypothalamic insulin signalling.
Material and methods:
Using DPPH and ABTS assays, total phenolic content in bilberry fruit and its antioxidant activities were examined. The selected biochemical parameters of blood (glucose, fructosamine, total cholesterol, HDL-cholesterol, LDL-cholesterol and triglycerides), as well as the expression of insulin receptors, were studied in the hippocampal CA1 field of healthy and diabetic (streptozotocin-induced; 60 mg kg-1 body weight) Wistar rats fed with bilberry fruit (16 g kg-1 body weight per day; 6 weeks), as well as of the corresponding control groups.
Results:
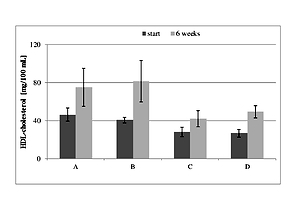
Biochemical analyses revealed ambiguous results, but a significantly (P<0.05) decrease in the level of LDL-cholesterol was observed in the group of healthy rats supplemented with bilberry pulp after 6 weeks of the treatment. There was also a difference (P<0.05) in the level of LDL-cholesterol in the mentioned healthy animals fed with bilberry, versus the healthy control group. An increased number of insulin receptors-immunoreactive neurons as well as nerve fibres in the CA1 field of diabetic rats fed with bilberry fruit was also found.
Conclusions:
An inclusion of bilberry fruit in the daily diet during the course of diabetes can lead to plasticity of hippocampal neurons/nerve fibres, manifested by changes in insulin receptors expression. Whether or not the observed changes had protective effects (by reducing damages caused by diabetes mellitus) on the function of the central nervous system neurons needs further study.
Bilberry fruit is believed to be a promising factor in the treatment of diabetes mellitus. Chronic hyperglycaemia affects the function of the central nervous system, which may be manifested as changes in hypothalamic insulin signalling.
Material and methods:
Using DPPH and ABTS assays, total phenolic content in bilberry fruit and its antioxidant activities were examined. The selected biochemical parameters of blood (glucose, fructosamine, total cholesterol, HDL-cholesterol, LDL-cholesterol and triglycerides), as well as the expression of insulin receptors, were studied in the hippocampal CA1 field of healthy and diabetic (streptozotocin-induced; 60 mg kg-1 body weight) Wistar rats fed with bilberry fruit (16 g kg-1 body weight per day; 6 weeks), as well as of the corresponding control groups.
Results:
Biochemical analyses revealed ambiguous results, but a significantly (P<0.05) decrease in the level of LDL-cholesterol was observed in the group of healthy rats supplemented with bilberry pulp after 6 weeks of the treatment. There was also a difference (P<0.05) in the level of LDL-cholesterol in the mentioned healthy animals fed with bilberry, versus the healthy control group. An increased number of insulin receptors-immunoreactive neurons as well as nerve fibres in the CA1 field of diabetic rats fed with bilberry fruit was also found.
Conclusions:
An inclusion of bilberry fruit in the daily diet during the course of diabetes can lead to plasticity of hippocampal neurons/nerve fibres, manifested by changes in insulin receptors expression. Whether or not the observed changes had protective effects (by reducing damages caused by diabetes mellitus) on the function of the central nervous system neurons needs further study.
REFERENCES (47)
1.
Dragan S, Andrica F, Serban M-C, et al. Polyphenols-rich natural products for treatment of diabetes. Curr Med Chem. 2015; 22(1): 14–22. doi: 10.2174/0929867321666140826115422.
2.
Sidorova Y, Shipelin V, Mazo V, et al. Hypoglycemic and hypolipidemic effect of Vaccinium myrtillus L. leaf and Phaseolus vulgaris L. seed coat extracts in diabetic rats. Nutrition. 2017; 41: 107–112. doi: 10.1016/j.nut.2017.04.010.
3.
Hoggard N, Cruickshank M, Moar K, et al. A single supplement of a standardised bilberry (Vaccinium myrtillus L.) extract (36 % wet weight anthocyanins) modifies glycaemic response in individuals with type 2 diabetes controlled by diet and lifestyle. J Nutr Sci. 2013; 2: e22. doi: 10.1017/jns.2013.16. eCollection 2013.
4.
Ştefănescu (Braic) R, Vari C, Imre S, et al. Vaccinium extracts as modulators in experimental type 1 diabetes. J Med Food. 2018; 21(11): 1106–1112. doi: 10.1089/jmf.2017.0141.
5.
Törrönen R, Kolehmainen M, Sarkkinen E, et al. Berries reduce postprandial insulin responses to wheat and rye breads in healthy women. J Nutr. 2013; 143(4): 430–436. doi: 10.3945/jn.112.169771.
6.
Ancillotti C, Ciofi L, Rossini D, et al. Liquid chromatographic/electrospray ionization quadrupole/time of flight tandem mass spectrometric study of polyphenolic composition of different Vaccinium berry species and their comparative evaluation. Anal Bioanal Chem. 2017; 409(5): 1347–1368. doi: 10.1007/s00216-016-0067-y.
7.
Ştefanuţ MN, Cata A, Pop R, et al. Anti-hyperglycemic effect of bilberry, blackberry and mulberry ultrasonic extracts on diabetic rats. Plant Foods Hum Nutr. 2013; 68(4): 378–384. doi: 10.1007/s11130-013-0380-y.
8.
Varut RM, Gîrd CE, Rotaru LT, et al. Evaluation of polyphenol and flavonoid profiles and the antioxidant effect of Carduus acanthoides hydroalcoholic extract compared with Vaccinium myrtillus in an animal model of diabetes mellitus. Pharm Chem J. 2018; 51(879): 1088–1095. doi: 10.1007/s11094-018-1746-0.
9.
Borowiec K, Szwajgier D, Targoński Z, et al. Cholinesterase inhibitors isolated from bilberry fruit. J Funct Foods. 2014; 11: 313–321. doi: 10.1016/j.jff.2014.10.008.
10.
Szwajgier D, Borowiec K. Screening for cholinesterase inhibitors in selected fruits and vegetables. Electron J Pol Agric Univ. 2012; 15(2): 6.
11.
Nguyen TT, Ta QTH, Nguyen TKO, et al. Type 3 diabetes and its role implications in Alzheimer’s disease. Int J Mol Sci. 2020; 21(9): 3165. doi: 10.3390/ijms21093165.
12.
Ferreira LSS, Fernandes CS, Vieira MNN, et al. Insulin resistance in Alzheimer’s Disease. Front Neurosci. 2018; 12: 830. doi: 10.3389/fnins.2018.00830.
13.
Moheet A, Mangia S, Seaquist ER. Impact of diabetes on cognitive function and brain structure. Ann NY Acad Sci. 2015; 1353: 60–71. doi: 10.1111/nyas.12807.
14.
Ho N, Sommers MS, Lucki I. Effects of diabetes on hippocampal neurogenesis: links to cognition and depression. Neurosci Biobehav Rev. 2013; 37(8): 1346–1362. doi: 10.1016/j.neubiorev.2013.03.010.
15.
Pomytkin I, Costa-Nunes JP, Kasatkin V, et al. Insulin receptor in the brain: Mechanisms of activation and the role in the CNS pathology and treatment. CNS Neurosci Ther. 2018; 24(9): 763–774. doi: 10.1111/cns.12866.
16.
Soto M, Cai W, Konishi M, et al. Insulin signaling in the hippocampus and amygdala regulates metabolism and neurobehavior. Proc Natl Acad Sci U S A. 2019; 116(13): 6379–6384. doi: 10.1073/pnas.1817391116.
17.
Banks WA, Owen JB, Erickson MA. Insulin in the brain: there and back again. Pharmacol Ther. 2012; 136(1): 82–93. doi: 10.1016/j.pharmthera.2012.07.006.
18.
De Felice FG, Benedict C. A key role of insulin receptors in memory. Diabetes. 2015; 64(11): 3653–3655. doi: 10.2337/dbi15-0011.
19.
Bobinaitė R, Viškelis P, Venskutonis PR. Variation of total phenolics, anthocyanins, ellagic acid and radical scavenging capacity in various raspberry (Rubus spp.) cultivars. Food Chem. 2012; 132(3): 1495–1501. doi: 10.1016/j.foodchem.2011.11.137.
20.
Brand-Williams W, Cuvelier ME, Berset C. Use of free radical method to evaluate antioxidant activity. Lebensm-Wiss Technol. 1995; 28(1): 2525–2530. doi: 10.1016/S0023-6438(95)80008-5.
21.
Miller NJ, Rice-Evans C, Davies MJ, et al. A novel method for measuring antioxidant capacity and its application to monitoring the antioxidant status in premature neonates. Clin Sci (Lond). 1993; 84(4): 407–412. doi: 10.1042/cs0840407.
22.
Chandirasegaran G, Elanchezhiyan C, Ghosh K, et al. Berberine chloride ameliorates oxidative stress, inflammation and apoptosis in the pancreas of streptozotocin induced diabetic rats. Biomed Pharmacother. 2017; 95: 175–185. doi: 10.1016/j.biopha.2017.08.040.
23.
Masuda K, Aizawa N, Watanabe D, et al. Pathophysiological changes of the lower urinary tract behind voiding dysfunction in streptozotocin-induced long-term diabetic rats. Sci Rep. 2020; 10: 4182. doi: 10.1038/s41598-020-61106-y.
24.
Nair AB, Jacob S. A simple practice guide for dose conversion between animals and human. J Basic Clin Pharm. 2016; 7(2): 27–31. doi: 10.4103/0976-0105.177703.
25.
Matysek M, Krakowska I, Lonc G, et al. Expression of α and β oestrogen receptors within the claustrum in rabbit males. Bull Vet Inst Pulawy. 2014; 58(1): 157–161. doi: 10.2478/bvip-2014-0024.
26.
Kunachowicz H, Przygoda B, Nadolna I, et al. Tabele składu i wartości odżywczej żywności. 4th ed. Wydawnictwo Lekarskie PZWL, 2017.
27.
Zhou Q, Wu J, Tang J, et al. Beneficial effect of higher dietary fiber intake on plasma HDL-C and TC/HDL-C ratio among Chinese rural-to-urban migrant workers. Int J Environ Res Public Health. 2015; 12(5): 4726–4738. doi: 10.3390/ijerph120504726.
28.
Aaby K, Grimmer S, Holtung L. Extraction of phenolic compounds from bilberry (Vaccinium myrtillus L.) press residue: Effects on phenolic composition and cell proliferation. LWT-Food Sci Technol. 2013; 54(1): 257–264. doi: 10.1016/j.lwt.2013.05.031.
29.
Tian Y, Liimatainen J, Alanne AL, et al. Phenolic compounds extracted by acidic aqueous ethanol from berries and leaves of different berry plants. Food Chem. 2017; 220: 266–281. doi: 10.1016/j.foodchem.2016.09.145.
30.
Hidalgo G-I, Almajano MP. Red fruits: Extraction of antioxidants, phenolic content, and radical scavenging determination: A review. Antioxidants (Basel). 2017; 6(1): 7. doi: 10.3390/antiox6010007.
31.
Mikulic-Petkovsek M, Schmitzer V, Slatnar A, et al. A comparison of fruit quality parameters of wild bilberry (Vaccinium myrtillus L.) growing at different locations. J Sci Food Agric. 2015; 95(4): 776–785. doi: 10.1002/jsfa.6897.
32.
Kolehmainen M, Mykkänen O, Kirjavainen PV, et al. Bilberries reduce low-grade inflammation in individuals with features of metabolic syndrome. Mol Nutr Food Res. 2012; 56(10): 1501–1510. doi: 10.1002/mnfr.201200195.
33.
Kim J, Kim CS, Lee YM, et al. Vaccinium myrtillus extract prevents or delays the onset of diabetes-induced blood-retinal barrier breakdown. Int J Food Sci Nutr. 2015; 66(2): 236–242. doi: 10.3109/09637486.2014.979319.
34.
Roghani M, Baluchnejadmojarad T, Taheri S. The effect of feeding with aerial part of Vaccinium myrtillus on blood glucose and lipids of diabetic rats. Iran J Diabetes Lipid Disord. 2007; 7: 151–158.
35.
Asgary S, RafieianKopaei M, Sahebkar A, et al. Anti-hyperglycemic and anti-hyperlipidemic effects of Vaccinium myrtillus fruit in experimentally induced diabetes (antidiabetic effect of Vaccinium myrtillus fruit). J Sci Food Agric. 2016; 96(3): 764–768. doi: 10.1002/jsfa.7144.
36.
Madihi Y, Merrikhi A, Baradaran A, et al. Bioactive components and the effect of hydroalcoholic extract of Vaccinium myrtillus on postprandial atherosclerosis risk factors in rabbits. Pak J Med Sci. 2013; 29(1): 384–389. doi: 10.12669/pjms.291(Suppl).3539.
37.
Habanova M., Saraiva JA, Haban M, et al. Intake of bilberries (Vaccinium myrtillus L.) reduced risk factors for cardiovascular disease by inducing favorable changes in lipoprotein profiles. Nutr Res. 2016; 36(12): 1415–1422. doi: 10.1016/j.nutres.2016.11.010.
38.
Crespo MC, Visioli F. A brief review of blue- and bilberries’ potential to curb cardio-metabolic perturbations: Focus on diabetes. Curr Pharm Des. 2017; 23(7): 983–988. doi: 10.2174/1381612822666161010120523.
39.
Matysek M, Mozel S, Szalak R, et al. Effect of feeding with bilberry fruit on the expression pattern of αCaMKII in hippocampal neurons in normal and diabetic rats. Pol J Vet Sci. 2017; 20(2): 313–319. doi: 10.1515/pjvs-2017-0038.
40.
Vogt MC, Brüning JC. CNS insulin signaling in the control of energy homeostasis and glucose metabolism- from embryo to old age. Trends Endocrinol Metab. 2013; 24(2): 76–84. doi: 10.1016/j.tem.2012.11.004.
41.
Jayaraj RL, Azimullah S, Beiram R. Diabetes as a risk factor for Alzheimer’s disease in the Middle East and its shared pathological mediators. Saudi J Biol Sci. 2020; 27(2): 736–750. doi: 10.1016/j.sjbs.2019.12.028.
42.
Stranahan AM. Models and mechanisms for hippocampal dysfunction in obesity and diabetes. Neuroscience. 2015; 19(309): 125–139. doi: 10.1016/j.neuroscience.2015.04.045.
43.
Derakhshan F, Toth C. Insulin and the brain. Curr Diabetes Rev. 2013; 9(2): 102–116.
44.
Zhang G, Fang H, Li Y, et al. Neuroprotective effect of astragalus polysacharin on streptozotocin (STZ)-induced diabetic rats. Med Sci Monit. 2019; 25: 135–141. doi: 10.12659/MSM.912213.
45.
Infante-Garcia C, Ramos-Rodriguez JJ, Galindo-Gonzalez L, et al. Long-term central pathology and cognitive impairment are exacerbated in a mixed model of Alzheimer’s disease and type 2 diabetes. Psychoneuroendocrinology. 2016; 65: 15–25. doi: 10.1016/j.psyneuen.2015.12.001.
46.
Grillo CA, Woodruff JL, Macht VA et al. Insulin resistance and hippocampal dysfunction: Disentangling peripheral and brain causes from consequences. Exp Neurol. 2019; 318: 71–77. doi: 10.1016/j.expneurol.2019.04.012.
47.
Subash S, Essa MM, Al-Adawi S, et al. Neuroprotective effects of berry fruits on neurodegenerative diseases. Neural Regen Res. 2014; 9(16): 1557–66. doi: 10.4103/1673-5374.139483.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.