Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
RESEARCH PAPER
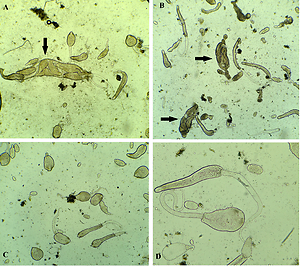
Phenoloxidase is involved in the immune reaction of Helix lucorum to parasitic infestation by dicrocoeliid trematode
1
Veterinary Division, Vocational School of Gevas, Van Yuzuncu Yil University, Van, Turkey
2
Department of Parasitology, Faculty of Medicine, Van Yuzuncu Yil University, Van, Turkey
Corresponding author
Ahmet Hakan Unlu
Veterinary Division, Vocational School of Gevas, Yuzuncu Yil University, Van, Turkey
Veterinary Division, Vocational School of Gevas, Yuzuncu Yil University, Van, Turkey
Ann Agric Environ Med. 2021;28(3):426-429
KEYWORDS
TOPICS
ABSTRACT
Introduction:
Phenoloxidases are known to play a role in the immune defences of arthropods and molluscs. In the invertebrates, phenoloxidases mediate three major physiologically important processes: sclerotization, wound healing, and defence reactions. Helix lucorum serve as the first intermediate host for the larval stages of dicrocoeliid trematodes which infects animals as well as human beings.
Objective:
The aim of the study is to investigate the effect of larval forms of dicrocoeliid trematodes to phenoloxidase acitivity in H. lucorum, Linneaus, 1758, in Bitlis, Turkey. The effect of the snail’s shell colour to phenoloxidase activity was also investigated.
Material and methods:
Land snails (n=200) were collected by hand from their natural habitats during the period May – June 2019 in Bitlis, Turkey. Evaluation of the process was performed by measuring immune reaction of the snails against larval forms of dicrocoeliid trematodes. Phenoloxidase activity assay was carried out using a spectrophotometer device based on 3,4-Dihydroxy-L-phenylalanine (L-dopa) hydrolysis.
Results:
The natural infection rate of the land snails with the developmental stages of dicrocoeliid trematodes was 20%. Phenoloxidase activity was found to be significantly higher (*p<0.05) in larval forms of dicrocoeliid trematodes infected snails when compared with non-infected snails. No effect of shell colours to phenoloxidase activity was observed.
Conclusions:
To the best of the authors’ knowledge, this study is the first to report that the phenoloxidase system is involved in the immune reaction of Helix lucorum to parasitic infestation by larval forms of dicrocoeliid trematodes.
Phenoloxidases are known to play a role in the immune defences of arthropods and molluscs. In the invertebrates, phenoloxidases mediate three major physiologically important processes: sclerotization, wound healing, and defence reactions. Helix lucorum serve as the first intermediate host for the larval stages of dicrocoeliid trematodes which infects animals as well as human beings.
Objective:
The aim of the study is to investigate the effect of larval forms of dicrocoeliid trematodes to phenoloxidase acitivity in H. lucorum, Linneaus, 1758, in Bitlis, Turkey. The effect of the snail’s shell colour to phenoloxidase activity was also investigated.
Material and methods:
Land snails (n=200) were collected by hand from their natural habitats during the period May – June 2019 in Bitlis, Turkey. Evaluation of the process was performed by measuring immune reaction of the snails against larval forms of dicrocoeliid trematodes. Phenoloxidase activity assay was carried out using a spectrophotometer device based on 3,4-Dihydroxy-L-phenylalanine (L-dopa) hydrolysis.
Results:
The natural infection rate of the land snails with the developmental stages of dicrocoeliid trematodes was 20%. Phenoloxidase activity was found to be significantly higher (*p<0.05) in larval forms of dicrocoeliid trematodes infected snails when compared with non-infected snails. No effect of shell colours to phenoloxidase activity was observed.
Conclusions:
To the best of the authors’ knowledge, this study is the first to report that the phenoloxidase system is involved in the immune reaction of Helix lucorum to parasitic infestation by larval forms of dicrocoeliid trematodes.
Ahmet Hakan Unlu, Abdurrahman Ekici. Phenoloxidase is involved in the immune reaction of Helix lucorum to parasitic infestation by dicrocoeliid trematode. Ann Agric Environ Med. Doi: 10.26444/aaem/140319
REFERENCES (34)
1.
Cerenius L, Söderhäll K. Immune properties of invertebrate phenoloxidases. Dev Comp Immunol. 2021; 104098. https://doi.org/10.1016/j.dci.....
2.
Lu A, Zhang Q, Zhang Q, et al. Insect prophenoloxidase: the view beyond immunity. Front Physiol. 2014; 5: 252. https://doi.org/10.3389/fphys.....
3.
Stączek S, Grygorczuk K, Zdybicka-Barabas A, et al. Different faces of phenoloxidase in animals. Postepy Biochemi. 2017; 63(4): 315–325.
4.
Jukanti A. Function(s)/Role(s) of Polyphenol Oxidases. In: Polyphenol Oxidases (PPOs) in Plants. Singapore: Springer; 2017. https://doi.org/10.1007/978-98....
5.
Le Clec’h W, Anderson TJ, Chevalier FD. Characterization of haemolymph phenoloxidase activity in two Biomphalaria snail species and impact of Schistosoma mansoni infection. Parasites Vectors. 2016; 9(1): 1–11. https://doi.org/10.1186/s13071....
6.
Leicht K, Jokela J, Seppälä O. An experimental heat wave changes immune defense and life history traits in a freshwater snail. Ecol Evol. 2013; 3(15): 4861–4871. https://doi.org/10.1002/ece3.8....
7.
Quinn EA, Malkin SH, Rowley AF, et al. Laccase and catecholoxidase activities contribute to innate immunity in slipper limpets, Crepidula fornicata. Dev Comp Immunol. 2020; 110: 103724. https://doi.org/10.1016/j.dci.....
8.
De Melo ES, Brayner FA, Junior NCP, et al. Investigation of defense response and immune priming in Biomphalaria glabrata and Biomphalaria straminea, two species with different susceptibility to Schistosoma mansoni. Parasitol Res. 2020; 119(1): 189–201. https://doi.org/10.1007/s00436....
9.
Wang L, Song X, Song L. The oyster immunity. Dev Comp Immunol. 2018; 80: 99–118. https://doi:10.1016/j.dci.2017....
10.
Noothuan N, Amparyup P, Tassanakajon A. Melanization inhibition protein of Penaeus monodon acts as a negative regulator of the prophenoloxidase-activating system. Dev Comp Immunol. 2017; 72: 97–102. https://doi.org/10.1016/j.dci.....
11.
Cerenius L, Lee BL, Söderhäll K. The proPO-system: pros and cons for its role in invertebrate immunity. Trends Immunol. 2008; 29(6): 263–271. https://doi.org/10.1016/j.it.2....
12.
Pojmańska, T. Family Dicrocoeliidae Looss, 1899. Keys to The Trematoda Volume. 2008; 3: 233–260. https://doi.org/10.1079/978085....
13.
Olsen OW. Animal parasites: their life cycles and ecology. New York: Dover Publishing; 1986.
14.
Otranto D, Traversa D. A review of dicrocoeliosis of ruminants including recent advances in the diagnosis and treatment. Vet Parasitol. 2002; 107(4): 317–335. https://doi.org/10.1016/s0304-....
15.
Conte R. Heliciculture: purpose and economic perspectives in the European community. IST Journal, 2015.
16.
Gliński Z, Jarosz J. Molluscan immune defenses. Arch Immunol Ther Exp. 1997; 45(2–3): 149–155.
17.
Schütt H. Turkish Land Snails, 1758–2000. Vollständig Revidierte und Erweiterte Auflage, Natur & Wissenschaft, 2005.
18.
Renwrantz L, Schäncke W, Harm H, et al. Discriminative ability and function of the immunobiological recognition system of the snail Helix pomatia. J Comp Physiol. 1981; 141(4): 477–488.
19.
Segun AO. Land snails (Dissection guides of common tropical animals). Ethiope Publications, 1973.
20.
Seppälä O, Jokela J. Maintenance of genetic variation in immune defense of a freshwater snail: role of environmental heterogeneity. Evolution. 2010; 64(8): 2397–2407. https://doi.org/10.1111/j.1558....
21.
Rajendran S, Vasudevan S. Activation of prophenoloxidase and hyperglycemia as indicators of microbial stress in the blue swimmer crab Portunus pelagicus. Mar Pollut Bull. 2020; 160: 111711. https://doi.org/10.1016/j.marp....
22.
Nakhleh J, El Moussawi L, Osta MA. The melanization response in insect immunity. Adv Insect Physiol. 2017; 52: 83–109. https://doi.org/10.1016/bs.aii....
23.
Yildirim MZ, Kebapci U, Gumus BA. Edible snails (Terrestrial) of Turkey. Turk J Zool. 2004; 28(4): 329–335.
24.
Eser M, Kartal K, Navruz FZ. The Prevalence Of Dicrocoeliidae (Digenea) Larval Stages In The First Intermediate Host Helix Lucorum,
1758 In Eskisehir And Bartin Provinces Of Turkey. Eskisehir Tech Univ J Sci Tech. 2021; 10(1): 38–43. https://doi.org/10.18036/estub...
25.
Kartal K, Mustafa K, Mustafa E. The Prevalance of larval stages of small liver fluke Dicrocoelium dendriticum in the first intermediate host Helix lucorum Linnaeus, 1758 (Mollusca: Pulmonata) in Afyonkarahisar district. Kocatepe Vet J. 2015; 8(1): 51–55. https://doi.org/10.18036/estub....
26.
Unlu AH, Bilgic HB, Eren H, Karagenc T. Prevalence of Larval-Stage Dicrocoeliidae (Digenea) Trematodes in Helix lucorum (Mollusca: Pulmonata) in Van Province. Turk J Parasitology. 2017; 41(4): 204–207. https://doi.org/10.5152/tpd.20....
27.
Gerdol M, Gomez-Chiarri M, Castillo MG, et al. Immunity in molluscs: recognition and effector mechanisms, with a focus on bivalvia. In Advances in Comparative Immunology. Cham: Springer; 2018. p. 225–341. https://doi.org/10.1007/978-3-....
28.
Zhou S, Zhao L, Zhao T. Phenol oxidase activity of Oncomelania snails in different ages and seasons. Chin J Zoonoses. 2010; 26(9): 856–861.
29.
Amparyup P, Charoensapsri W, Tassanakajon A. Prophenoloxidase system and its role in shrimp immune responses against major pathogens. Fish Shellfish Immunol. 2013; 34(4): 990–1001. https://doi.org/10.1016/j.fsi.....
30.
Quinn EA, Malkin SH, Rowley AF, et al. Laccase and catecholoxidase activities contribute to innate immunity in slipper limpets, Crepidula fornicata. Dev Comp Immunol. 2020; 110: 103725. https://doi.org/10.1016/j.dci.....
31.
Ito S, Konuma J. Disruptive selection of shell colour in land snails: a mark–recapture study of Euhadra peliomphala simodae. Biol J Linn Soc. 2020; 129(2): 323–333. https://doi.org/10.1093/biolin....
32.
Saenko SV, Schilthuizen M. Evo-devo of shell colour in gastropods and bivalves. Curr Opin Genet Dev. 2021; 69: 1–5. https://doi.org/10.1016/j.gde.....
33.
Scheil AE, Hilsmann S, Triebskorn R, et al. Shell colour polymorphism, injuries and immune defense in three helicid snail species, Cepaea hortensis, Theba pisana and Cornu aspersum maximum. Results Immunol. 2013; 3: 73–78. https://doi.org/10.1016/j.rini....
34.
Scheil AE, Hilsmann S, Triebskorn R, et al. Shell colouration and parasite tolerance in two helicoid snail species. J Invertebr Pathol. 2014; 117: 1–8. https://doi.org/ 10.1016/j.jip.2014.01.003.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.