Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
RESEARCH PAPER
Improved efficacy of eugenol and trans-anethole in combination with octenidine dihydrochloride against Candida albicans and Candida parapsilosis
1
Department of Allergology and Respiratory Rehabilitation; Medical University, Łódź, Poland
2
Department of Diagnostic Immunology; Pomeranian Medical University, Szczecin, Poland
3
Pharmaceutical Biology; Institute of Pharmacy; University of Greifswald, Germany
4
Centre of Bioimmobilisation and Innovative Packaging Materials; Faculty of Food Sciences and Fisheries; West Pomeranian University of Technology, Szczecin, Poland
5
Pharmaceutical Biotechnology Department; Medical University, Łódź, Poland
6
Department of Laboratory Medicine; Pomeranian Medical University in Szczecin, Poland
7
Department of Pharmaceutical Microbiology and Microbiological Diagnostic, Medical University, Łódź, Poland
Corresponding author
Monika Sienkiewicz
Department of Pharmaceutical Microbiology and Microbiological Diagnostic, Medical University of Lodz, Muszyńskiego 1, 90-151, Lodz, Poland
Department of Pharmaceutical Microbiology and Microbiological Diagnostic, Medical University of Lodz, Muszyńskiego 1, 90-151, Lodz, Poland
Ann Agric Environ Med. 2023;30(1):204-210
KEYWORDS
TOPICS
- Biological agents posing occupational risk in agriculture, forestry, food industry and wood industry and diseases caused by these agents (zoonoses, allergic and immunotoxic diseases)
- State of the health of rural communities depending on various factors: social factors, accessibility of medical care, etc.
ABSTRACT
Introduction and objective:
Candidiasis is a fungal infection caused by yeasts from the Ogenus Candida. Considering increasing antifungal resistance rates the activity was analyzed of natural compounds to eradicate Candida spp. The aim of the study was to check the antifungal activity of selected essential oil compounds (EOCs; thymol, menthol, eugenol [E], carvacrol, trans-anethole [TA]) alone, and in combination with octenidine dihydrochloride (OCT) against C. albicans and C. parapsilosis reference, and clinical strains.
Material and methods:
Investigated clinical isolates were obtained from skin wounds of patients treated for superficial wounds candidiasis. The following parameters were studied: antifungal susceptibility testing using the VITEK system, antifungal activity of EOCs alone and in combination with OCT using microdilution and checkerboard assays, antifungal efficacy of selected chemicals using time-kill curve assay, and changes in cell permeability in the presence of selected chemicals using crystal violet assay.
Results:
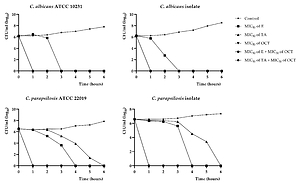
Clinical isolates of C. albicans and C. parapsilosis were resistant to fluconazole and voriconazole. The highest inhibition activity against Candida isolates was observed for E. The OCT – TA and OCT – E combinations showed synergistic and additive activities against all strains, respectively. These combinations also appeared to affect the rate of yeast cell killing and increasing the permeability of Candida cells.
Conclusions:
The study indicates that E and TA potentially used in formulation with OCT might eradicate pathogenic yeasts; however, microbiological and clinical studies are still required.
Candidiasis is a fungal infection caused by yeasts from the Ogenus Candida. Considering increasing antifungal resistance rates the activity was analyzed of natural compounds to eradicate Candida spp. The aim of the study was to check the antifungal activity of selected essential oil compounds (EOCs; thymol, menthol, eugenol [E], carvacrol, trans-anethole [TA]) alone, and in combination with octenidine dihydrochloride (OCT) against C. albicans and C. parapsilosis reference, and clinical strains.
Material and methods:
Investigated clinical isolates were obtained from skin wounds of patients treated for superficial wounds candidiasis. The following parameters were studied: antifungal susceptibility testing using the VITEK system, antifungal activity of EOCs alone and in combination with OCT using microdilution and checkerboard assays, antifungal efficacy of selected chemicals using time-kill curve assay, and changes in cell permeability in the presence of selected chemicals using crystal violet assay.
Results:
Clinical isolates of C. albicans and C. parapsilosis were resistant to fluconazole and voriconazole. The highest inhibition activity against Candida isolates was observed for E. The OCT – TA and OCT – E combinations showed synergistic and additive activities against all strains, respectively. These combinations also appeared to affect the rate of yeast cell killing and increasing the permeability of Candida cells.
Conclusions:
The study indicates that E and TA potentially used in formulation with OCT might eradicate pathogenic yeasts; however, microbiological and clinical studies are still required.
REFERENCES (44)
1.
Manolakaki D, Velmahos G, Kourkoumpetis T, et al. Candida infection and colonization among trauma patients. Virulence. 2010;1(5):367–75. doi: 10.4161/viru.1.5.12796.
2.
Demiraslan H, Alabay S, Kilic AU, et al. Cutaneous candidiasis caused by Candida glabrata in a HIV/AIDS patient. Int J STD AIDS. 2013;24:753–5. doi: 10.1177/0956462413479897.
3.
Dabiri S, Shams-Ghahfarokhi M, Razzaghi-Abyaneh M. Comparative analysis of proteinase, phospholipase, hydrophobicity and biofilm forming ability in Candida species isolated from clinical specimens. J Mycol Med. 2018;28:437–42. doi: 10.1016/j.mycmed.2018.04.009.
4.
Lim CS-Y, Rosli R, Seow HF, et al. Candida and invasive candidiasis: back to basics. Eur J Clin Microbiol Infect Dis. 2012;31:21–31. doi: 10.1007/s10096-011-1273-3.
5.
Kühbacher A, Burger-Kentischer A, Rupp S. Interaction of Candida species with the skin. Microorganisms. 2017;5:32. doi: 10.3390/microorganisms5020032.
6.
Kreijkamp-Kaspers S, Hawke K, Guo L, et al. Oral antifungal medication for toenail onychomycosis. Cochrane Database Syst Rev. 2017;7:CD010031. doi: 10.1002/14651858.CD010031.pub2.
7.
Shemer A, Sakka N, Baran R, et al. Clinical comparison and complete cure rates of terbinafine efficacy in affected onychomycotic toenails. J Eur Acad Dermatology Venereol. 2015;29:521–6. doi: 10.1111/jdv.12609.
8.
Pfaller MA, Diekema DJ, Gibbs DL, et al. Results from the ARTEMIS DISK Global Antifungal Surveillance Study, 1997 to 2007: a 10.5-year analysis of susceptibilities of Candida species to fluconazole and voriconazole as determined by CLSI standardized disk diffusion. J Cinical Microbiol. 2010;48:1366–77. doi: 10.1128/JCM.02117-09.
9.
Espinel-Ingroff A, Arendrup M, Cantón E, et al. Multicenter study of method-dependent epidemiological cutoff values for detection of resistance in Candida spp. and Aspergillus spp. to amphotericin B and echinocandins for the Etest agar diffusion method. Antimicrob Agents Chemother. 2017;61. doi: 10.1128/AAC.01792-16.
10.
Yang Y-L, Wang A-H, Wang C-W, et al. Susceptibilities to amphotericin B and fluconazole of Candida species in Taiwan Surveillance of Antimicrobial Resistance of Yeasts 2006. Diagn Microbiol Infect Dis. 2008;61:175–80. doi: 10.1016/j.diagmicrobio.2008.01.011.
11.
Superti F, De Seta F. Warding off recurrent yeast and bacterial vaginal infections: Lactoferrin and lactobacilli. Microorganisms. 2020;8:130. doi: 10.3390/microorganisms8010130.
12.
Campos LF, Tagliari E, Casagrande TAC, et al. Effects of probiotics supplementation on skin wound healing in diabetic rats. Brazilian Arch Dig Surg. 2020;33:e1498. doi: 10.1590/0102-672020190001e1498.
13.
Vakilian K, Atarha M, Bekhradi R, et al. Healing advantages of lavender essential oil during episiotomy recovery: a clinical trial. Complement Ther Clin Pract. 2011;17:50–3. doi: 10.1016/j.ctcp.2010.05.006.
14.
Valdivieso-Ugarte M, Gomez-Llorente C, Plaza-Díaz J, et al. Antimicrobial, antioxidant, and immunomodulatory properties of essential oils: A systematic review. Nutrients. 2019;11:2786. doi: 10.3390/nu11112786.
15.
European Committee on Antimicrobial Susceptibility Testing. Breakpoint tables for interpretation of MICs for antifungal agents. Version 10.0, valid from 2020-02-04. n.d.
16.
Clinical and Laboratory Standards Institute (CLSI). M27-A3. Reference method for broth dilution antifungal susceptibility testing of yeasts. Approved standard 3th ed. Reference method for broth dilution 2008.
17.
Kalemba D, Kunicka A. Antibacterial and antifungal properties of essential oils. Curr Med Chem. 2003;10:813–29. doi: 10.2174/0929867033457719.
18.
Yap PSX, Lim SHE, Hu CP, et al. Combination of essential oils and antibiotics reduce antibiotic resistance in plasmid-conferred multidrug resistant bacteria. Phytomedicine. 2013;20:710–3. doi: 10.1016/j.phymed.2013.02.013.
19.
Kang J, Liu L, Wu X, et al. Effect of thyme essential oil against Bacillus cereus planktonic growth and biofilm formation. Appl Microbiol Biotechnol. 2018;102:10209–18. doi: 10.1007/s00253-018-9401-y.
20.
Devi KP, Nisha SA, Sakthivel R, et al. Eugenol (an essential oil of clove) acts as an antibacterial agent against Salmonella Typhi by disrupting the cellular membrane. J Ethnopharmacol. 2010;130:107–15. doi: 10.1016/j.jep.2010.04.025.
21.
Latifah-Munirah B, Himratul-Aznita WH, Zain NM. Eugenol, an essential oil of clove, causes disruption to the cell wall of Candida albicans (ATCC 14053). Front Life Sci. 2015;8:231–40. doi: 10.1080/21553769.2015.1045628.
22.
Findley K, Oh J, Yang J, et al. Topographic diversity of fungal and bacterial communities in human skin. Nature. 2013;498:367–70. doi: 10.1038/nature12171.
23.
Thomas-Rüddel DO, Schlattmann P, Pletz M, et al. Risk factors for invasive Candida infection in critically ill patients: A systematic review and meta-analysis. Chest. 2022;161:345–55. doi: 10.1016/j.chest.2021.08.081.
24.
Flowers SA, Colón B, Whaley SG, et al. Contribution of clinically derived mutations in ERG11 to azole resistance in Candida albicans. Antimicrob Agents Chemother. 2015;59:450–60. doi: 10.1128/AAC.03470-14.
25.
Coste AT, Karababa M, Ischer F, et al. TAC1, transcriptional activator of CDR genes, is a new transcription factor involved in the regulation of Candida albicans ABC transporters CDR1 and CDR2. Eukaryot Cell. 2004;3:1639–52. doi: 10.1128/EC.3.6.1639-1652.2004.
26.
Branco J, Silva AP, Silva RM, et al. Fluconazole and voriconazole resistance in Candida parapsilosis is conferred by gain-of-function mutations in MRR1 transcription factor gene. Antimicrob Agents Chemother. 2015;59:6629–33. doi: 10.1128/AAC.00842-15.
27.
Jones IA, Joshi LT. Biocide use in the antimicrobial era: A review. Molecules. 2021;26:2276. doi: 10.3390/molecules26082276.
28.
Meade E, Slattery MA, Garvey M. Biocidal resistance in clinically relevant microbial species: A major public health risk. Pathogens. 2021;10:598. doi: 10.3390/pathogens10050598.
29.
Cowen LE, Sanglard D, Howard SJ, et al. Mechanisms of antifungal drug resistance. Cold Spring Harb Perspect Med. 2014;5:a019752. doi: 10.1101/cshperspect.a019752.
30.
Ozturk A, Abdulmajed O, Aydin M. Investigation of antifungal, antibiofilm and anti-filamentation activities of biocides against Candida isolates. Ann Med Res. 2021;27:2041–2046.
31.
Cuellar-Rufino S, Arroyo-Xochihua O, Salazar-Luna A, et al. Iodine induces toxicity against Candida albicans and Candida glabrata through oxidative stress. Iran J Microbiol. 2022;14:260–7. doi: 10.18502/ijm.v14i2.9195.
32.
Ali B, Al-Wabel NA, Shams S, et al. Essential oils used in aromatherapy: A systemic review. Asian Pac J Trop Biomed. 2015;5:601–11. doi: 10.1016/j.apjtb.2015.05.007.
33.
Ugalde Martinez UO, Rodriguez Urra AB, Ubegun Lizaso A. Phytosanitary composition comprising essential oils that potentiate antifungal activity. US 10,813,360 B2, 2020.
34.
Tomczykowa M, Wróblewska M, Winnicka K, et al. Novel gel formulations as topical carriers for the essential oil of Bidens tripartita for the treatment of candidiasis. Molecules. 2018;23:2517. doi: 10.3390/molecules23102517.
35.
Dąbrowska M, Zielińska-Bliźniewska H, Kwiatkowski P, et al. Inhibitory effect of eugenol and trans-anethole alone and in combination with antifungal medicines on Candida albicans clinical isolates. Chem Biodivers. 2021;18:e2000843. doi: 10.1002/cbdv.202000843.
36.
Sharifzadeh A, Khosravi AR, Shokri H, et al. Synergistic anticandidal activity of menthol in combination with itraconazole and nystatin against clinical Candida glabrata and Candida krusei isolates. Microb Pathog. 2017;107:390–6. doi: 10.1016/j.micpath.2017.04.021.
37.
Swetha TK, Vikraman A, Nithya C, et al. Synergistic antimicrobial combination of carvacrol and thymol impairs single and mixed-species biofilms of Candida albicans and Staphylococcus epidermidis. Biofouling. 2020;36:1256–71. doi: 10.1080/08927014.2020.1869949.
38.
Spettel K, Bumberger D, Camp I, et al. Efficacy of octenidine against emerging echinocandin-, azole- and multidrug-resistant Candida albicans and Candida glabrata. J Glob Antimicrob Resist. 2022;29:23–8. doi: 10.1016/j.jgar.2022.01.028.
39.
Ponnachan P, Vinod V, Pullanhi U, et al. Antifungal activity of octenidine dihydrochloride and ultraviolet-C light against multidrug-resistant Candida auris. J Hosp Infect. 2019;102:120–4. doi: 10.1016/j.jhin.2018.09.008.
40.
Tariq S, Wani S, Rasool W, et al. A comprehensive review of the antibacterial, antifungal and antiviral potential of essential oils and their chemical constituents against drug-resistant microbial pathogens. Microb Pathog. 2019;134:103580. doi: 10.1016/j.micpath.2019.103580.
41.
Hardy K, Sunnucks K, Gil H, et al. Increased usage of antiseptics is associated with reduced susceptibility in clinical isolates of Staphylococcus aureus. MBio. 2018;9:e00894–18. doi: 10.1128/mBio.00894-18.
42.
Kegley S, Konlisk E, Moses M. Marin municipal water district. Herbicide risk assessment. Overview and Executive Summary. 2010;1:1–20.
43.
Jenner PM, Hagan EC, Taylor JM, et al. Food flavourings and compounds of related structure I. Acute oral toxicity. Food Cosmet Toxicol. 1964;2:327–43. doi: 10.1016/S0015-6264(64)80192-9.
44.
Lin FSD. trans-Anethole. In: Joint FAO/WHO Expert Committee on Food Additives. Toxicological evaluation of certain food additives and contaminants. WHO Food Additives Series 28. WHO Geneva. 1991;135–52.
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.