Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
RESEARCH PAPER
Costs of plasmocytic myeloma therapy in the drug programme at a Regional Oncology Centre in Poland
1
National Institute of Medicine of the Ministry of the Interior and Administration, Warsaw, Poland
2
Department of Clinical Haematology, Independent Public Health Care Institution of the Ministry of the Interior and
Administration Hospital (MSWiA) with Warmian-Masurian Cancer Centre, Olsztyn, Poland
3
National Health Fund, Warsaw, Poland
Corresponding author
Krystyna Futyma
National Institute of Medicine of the Ministry of the Interior and Administration, Poland
National Institute of Medicine of the Ministry of the Interior and Administration, Poland
Ann Agric Environ Med. 2023;30(4):705-714
KEYWORDS
TOPICS
ABSTRACT
Introduction:
The rapidly growing market for drugs, including oncology and haemato-oncology drugs, is generating enormous financial expenditure for healthcare systems. In Poland, access to high-cost treatments is possible mainly within drug programmes, funded by public healthcare systems. The path of proceeding adopted in Polish regulations is similar to the solutions adopted in other countries.
Objective:
The aim of this study was to demonstrate the actual costs incurred by the treatment entity in the process of treating patients under the drug programme at the Regional Oncology Centre in Olsztyn, north-east Poland.
Material and methods:
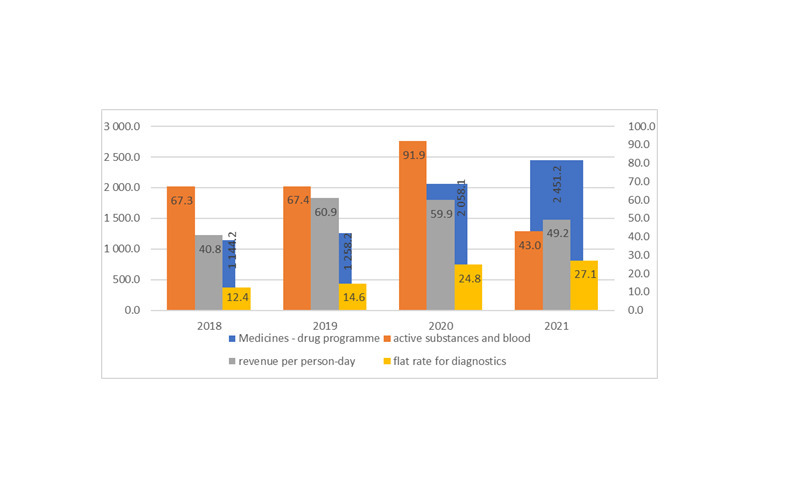
The oncology drug programme B.54 ‘Treatment of patients with refractory or malignant plasmocytic myeloma’ implemented at the Regional Oncology Centre in Poland between 2018–2021, was selected for the analysis. The choice of the B.54 programme was based on the small population of patients meeting the inclusion criteria for this programme, and the large number of diagnostic procedures stipulated in the drug programme description. On average, 25 patients were treated per year. The financial analysis used the financial categories related to hospital billing information. The costs were presented based on the purchasing power parity of money in 2021, i.e. 1 USD-inter is equivalent to 1.837 PLN.
Results:
The flat rate form of financing medical services does not cover the actual costs of treatment. Providing patients with necessary medical services without their full coverage by the public payer, burdens the budget of the centre and may lead to indebtedness of the treatment entity.
Conclusions:
Without an increase in the valuation of benefits under drug programmes, corresponding to the actual costs of treatment, the expected increase in access to innovative therapies will be difficult to accomplish.
The rapidly growing market for drugs, including oncology and haemato-oncology drugs, is generating enormous financial expenditure for healthcare systems. In Poland, access to high-cost treatments is possible mainly within drug programmes, funded by public healthcare systems. The path of proceeding adopted in Polish regulations is similar to the solutions adopted in other countries.
Objective:
The aim of this study was to demonstrate the actual costs incurred by the treatment entity in the process of treating patients under the drug programme at the Regional Oncology Centre in Olsztyn, north-east Poland.
Material and methods:
The oncology drug programme B.54 ‘Treatment of patients with refractory or malignant plasmocytic myeloma’ implemented at the Regional Oncology Centre in Poland between 2018–2021, was selected for the analysis. The choice of the B.54 programme was based on the small population of patients meeting the inclusion criteria for this programme, and the large number of diagnostic procedures stipulated in the drug programme description. On average, 25 patients were treated per year. The financial analysis used the financial categories related to hospital billing information. The costs were presented based on the purchasing power parity of money in 2021, i.e. 1 USD-inter is equivalent to 1.837 PLN.
Results:
The flat rate form of financing medical services does not cover the actual costs of treatment. Providing patients with necessary medical services without their full coverage by the public payer, burdens the budget of the centre and may lead to indebtedness of the treatment entity.
Conclusions:
Without an increase in the valuation of benefits under drug programmes, corresponding to the actual costs of treatment, the expected increase in access to innovative therapies will be difficult to accomplish.
REFERENCES (50)
1.
Web report. Beating cancer – the role of Europe’s environment. https://www.eea.europa.eu/publ... (access: 2022.07.08).
2.
Jakubiak K, Władysiuk M, Rutkowski J, et al. Raport. Nowe terapie w leczeniu chorych na nowotwory. Modern Healthcare Institute; 2022.
3.
Carlson JJ, Guzauskas GF, Chapman RH, et al. Cost-effectiveness of Drugs to Treat Relapsed/Refractory Multiple Myeloma in the United States. J Manag Care Spec Pharm. 2018;24(1):29–38. https://doi.org/10.18553/jmcp.....
4.
Giannopoulos K. Leczenie chorych na szpiczaka w Polsce jest coraz lepsze, ale wciąż nieoptymalne. https://pulsmedycyny.pl/leczen... (access: 2022.06.30).
5.
Xu T, Yang W, Chen L, et al. What are the implications of cost for myeloma therapy? Expert Rev Hematol. 2019;12(12):1005–1009. https://doi.org/10.1080/174740....
6.
Neves M, Trigo F, Rui B, et al. Multiple Myeloma in Portugal: Burden of Disease and Cost of Illness. Pharmaco Economics. 2021;39:579–587. https://doi.org/10.1007/s40273....
7.
Theroux H, Williams A, Liu M, et al. Multiple Myeloma Cost of Care Under the Oncology Care Model: The Influence of High-Cost Therapies. JCO Oncology Practice. 2020;16(10):1078–1084. https://doi.org/10.1200/JOP.19....
8.
Hus I, Drozd-Sokołowska J, Gil L, et al. Stosowanie leków biopodobnych w hematologii – stanowisko Polskiego Towarzystwa Hematologów i Transfuzjologów. Acta Haematol Pol. 2019;50(2):51–56. https://www.researchgate.net/p..., Stosowanie lekow biopodobnych w hematoonkologii – stanowisko Polskiego Towarzystwa Hematologów I Transfuzjologow (access: 2022.08.03).
9.
Hanning LH, Nielsen LK, Ibsen R, et al. The impact of changed treatment patterns in multiple myeloma on health-care utilisation and costs, myeloma complications, and survival: A population-based comparison between two time periods in Denmark. Eur J Haematol. 2021;107(1):63–73. https://doi.org/10.1111/ejh.13....
10.
Mikhael J, Ismaila N, Cheung MC, et al. Treatment of Multiple Myeloma: ASCO and CCO Joint Clinical Practice Guideline. J Clin Oncol. 2019;37(14):1228–1263. https://doi.org/10.1200/JCO.18....
11.
Asrar MM, Lad DP, Prinja S, et al. A systematic review of economic evaluations of treatment regimens in multiple myeloma. Expert Rev Pharmacoecon Outcomes Res. 2021;21(4):799–809. https://doi.org/10.1080/147371....
12.
Rajkumar VS. Value and Cost of Myeloma Therapy. ASCO Educational Book. 2018;38:662–666.
13.
Mailankody S, Prasad V. Implications of proposed medicare reforms to counteract high cancer drug prices. JAMA. 2016;316(3):271–272. https://doi.org/10.1001/jama.2....
14.
The Act of 12 May 2011 on the Reimbursement of Medicines, Foodstuffs for Particular Nutritional Uses and Medical Devices (Journal of Laws 2011, No. 122, item 696 as amended). https://isap.sejm.gov.pl/isap.... (access: 2022.06.14).
15.
Announcement of the Minister of Health of 21 February 2022 on the list of reimbursable medicines, foodstuffs for particular nutritional uses and medical devices as of 1 March 2022. https://www.gov.pl/web/zdrowie... (access: 2022.03.01).
16.
Wytyczne oceny technologii medycznych AOTMiT. https://www.aotm.gov.pl/media/..., Wytyczne AOTMiT.pdf (access: 2022.08.27).
18.
Dima D, Dower J, Comezo RL, et al. Evaluating Daratumumab in the Treatment of Multiple Myeloma: Safety, Efficacy and Place in Therapy. Cancer Manag Res. 2020;12:7891–7903. https://doi.org/10.2147/CMAR.S....
19.
Goel U, Usmani S, Kumar S. Current approaches to management of newly diagnosed multiple myeloma. Am J Hematol. 2022;97:3–25. https://doi.org/10.1002/ajh.26....
20.
Shah UA, Mailankody S. Emerging immunotherapies in multiple myeloma. BMJ. 2020;370:m3176. https://doi.org/10.1136/bmj.m3....
21.
Samuel JN, Booth CM, Eisenhauer E, et al. Association of Quality-of-Life Outcomes in Cancer Drug Trials With Survival Outcomes and Drug Class. JAMA Oncol. 2022;8(6):879–886. https://doi.org/10.1001/jamaon....
22.
Giebel S. Mamy dziś rewolucję w leczeniu wielu nowotworów krwi. https://zdrowie.wprost.pl/stre... (access: 2022.05.29).
23.
Hus I. Dostęp do nowych terapii w hematologii jest coraz lepszy, ale wciąż pojawiają się nowe potrzeby. https://www.mzdrowie.pl/leki/d... (access: 2022.08.04).
24.
Raport. Mechanizmy wczesnego dostępu do leków innowacyjnych na świecie ze szczególnym uwzględnieniem terapii onkologicznych. Warszawa: Instytut Zarządzania w Ochronie Zdrowia, Uczelnia Łazarskiego; 2016.
25.
Bach PB, Pearson SD. Payer and policy maker steps to support value-based pricing for drugs. JAMA. 2015;314(23):2503–2504. https://doi.org/10.1001/jama.2....
27.
MZ przygotowuje nową wersję programu lekowego dla chorych na szpiczaka plazmocytowego. https://pulsmedycyny.pl/mz-prz... (access: 2022.10.07).
28.
Blommestein HM, Franken MG, van Beurden-Tan CHV, et al. Cost-effectiveness of Novel Treatment Sequences for Transplant-Ineligible Patients With Multiple Myeloma. JAMA Netw Open. 2021;4(3):e213497. https://doi.org/10.1001/jamane....
29.
Fu S, Wu Ch-F, Wang M, et al. Cost Effectiveness of Transplant, Conventional Chemotherapy, and Novel Agents in Multiple Myeloma: A Systematic Review. Pharmacoeconomics. 2019;37(12):1421–1449. https://doi.org/10.1007/s40273....
30.
Order No. 75/2018/DGL of the President of the National Health Fund of 31 July October 2018 on lying down the terms and conditions for concluding and implementing contracts regarding hospital treatment in respect of drug programs. https://www.nfz.gov.pl/zarzadz... (access: 2022.05.30).
31.
Ailawadhi S, DerSarkissian M, Duh MS, et al. Cost Offsets in the Treatment Journeys of Patients With Relapsed/Refractory Multiple Myeloma. Clin Ther. 2019;41(3):477–493.e7. https://doi.org/10.1016/j.clin....
32.
Order No. 20/2021/DSOZ of the President of the National Health Fund of 27 January 2021 amending the order on detailed terms and conditions of contracts in the system of basic hospital healthcare benefits. https://www.nfz.gov.pl/zarzadz... zarzadzenia-prezesa-nfz/zarzadzenie-nr-202021dsoz,7307.html (access: 2022.06.04).
34.
Rajkumar SV, Dimopoulos MA, Palumbo A, et al. International Myeloma Working Group updated criteria for the diagnosis of multiple myeloma. Lancet Oncol. 2014;15(12):e538–548. https://doi.org/10.1016/S1470-....
35.
Kade G, Spaleniak S, Hałka J, et al. Monoclonal gammopathy of renal significance – diagnostic and therapeutic problems. OncoReview. 2020;4(40):139–144. https://doi.org/10.24292/01.OR....
36.
Giannopoulos K, Jamroziak K, Usnarska-Zubkiewicz L, et al. Zalecenia Polskiej Grupy Szpiczakowej dotyczące rozpoznawania i leczenia szpiczaka plazmocytowego oraz innych dyskrazji plazmocytowych na rok 2021.
37.
Giannopoulos K. Szpiczak z wysokim ryzykiem cytogenetycznym. https://pulsmedycyny.pl/szpicz... ysokim-ryzykiem-cytogenetycznym-992224 (access: 2022.06.30).
38.
Orlewska E. Reguły decyzyjne w ocenie ekonomicznej programów zdrowotnych. Farmakoekonomika. 2004;1.
39.
Patel KK, Giri S, Parker TL, et al. Cost-Effectiveness of First-Line Versus Second-Line Use of Daratumumab in Order, Transplant-Ineligible Patients With Multiple Myeloma. J Clin Oncol. 2021;39(10):1119–1128. https://doi.org/10.1200/JCO.20....
40.
Czech M, Gierczyński J, Jakubiak K, et al. Raport. Rozwój terapii lekowych w leczeniu chorych na nowotwory. Nowości. Innowacje. Przełomy. Modern Healthcare Institute; 2020.
41.
Stajszczyk M, Obarska I. Raport. Dostępność terapii i świadczeń w programach lekowych w chorobach autoimmunologicznych. Wpływ wprowadzenia ryczałtowego modelu opieki ambulatoryjnej na budżet płatnika publicznego. Warszawa: Health Care System Navigator; 2021.
42.
Iwańczuk T, Tomaszewska I, Wyszkowska A. Analiza instrumentów dzielenia ryzyka proponowanych we wnioskach refundacyjnych dla leków stosowanych w chorobach onkologicznych w ramach programów lekowych, przekazanych do AOTMiT w latach 2012–2018. Warszawa: Agencja Oceny Technologii Medycznych i Taryfikacji; 2019.
43.
Order No. 162/2020/DGL of the President of the National Health Fund of 16 October 2020 on lying down the terms and conditions for concluding and implementing contracts regarding hospital treatment in respect of drug programs. https://www.nfz.gov.pl/zarzadz... (access: 2022.06.12).
44.
Rozliczanie diagnostyki i leczenia nowotworów w praktyce – poradnik dla placówek medycznych. Kielce: Polskie Towarzystwo Koderów Medycznych; 2020. https://ptkm.org.pl/media/atta... k m _porad ni k _ rozlicza nie _d iagnost yk i _ i _ leczenia _nowotworow_w_praktyce.pdf (access: 2022.03.12).
45.
The Act of 27 August of 2004 on Healthcare Benefits Financed from Public Funds (Journal of Laws 2004, No 210, item 2135 as amended). https://isap.sejm.gov.pl/isap.... (access: 2022.07.14).
46.
The Act of 14 August of 2020 on Amending Certain Acts to Ensure the Functioning of Health Care in Connection with the COVID-19 Epidemic and after its Cessation (Journal of Laws 2020, item 1493). https://isap.sejm.gov.pl/isap.... (access: 2022.08.01).
47.
Stajszczyk M. Rozliczanie kosztów realizacji programów lekowych – ważna zmiana na czas epidemii COVID-19. https://www.rynekzdrowia.pl/Fi... (access: 2022.03.07).
48.
Gil J, Fontrier AM, Miracolo A, et al. Access to Personalised Oncology in Europe. The London School of Economics and Political Science; 2020.
49.
Terapie doustne poprawiają jakość życia pacjenta za szpiczakiem plazmocytowym. https://pulsmedycyny.pl/terapi... (access: 2022.05.29).
50.
Raport. Szpiczak plazmocytowy. Doświadczenia i oczekiwania w stosunku do metod leczenia. Hematoonkologia.pl, 2020.
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.