Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
REVIEW PAPER
Clinical evidence for the adaptogenic effects of Withania somnifera and Rhodiola rosea – A systematic review with molecular interpretation of psychometric outcomes
1
Department of Biology with Genetics, Medical University, Lublin, Poland
2
Department of Clinical Genetics, Medical University, Lublin, Poland
Corresponding author
Joanna Łuszczak
Department of Biology with Genetics, Medical University of Lublin, Chodzki 4a, 20-093, Lublin, Poland
Department of Biology with Genetics, Medical University of Lublin, Chodzki 4a, 20-093, Lublin, Poland
KEYWORDS
TOPICS
ABSTRACT
Introduction and objective:
Adaptogens are plant-derived substances that enhance the body’s resilience to physical and psychological stress, with Withania somnifera and Rhodiola rosea being among the most studied representatives. The aim of this review is to evaluate the adaptogenic effects of W. somnifera and R. rosea based on randomized controlled trials (RCTs).
Review methods:
A systematic literature search was performed using the key words: ‘ashwagandha’, ‘Withania somnifera’, ‘Rhodiola rosea’, and ‘plant adaptogen’. Twenty-four studies met the inclusion criteria – 19 on W. somnifera and 5 on R. rosea.
Brief description of the state of knowledge:
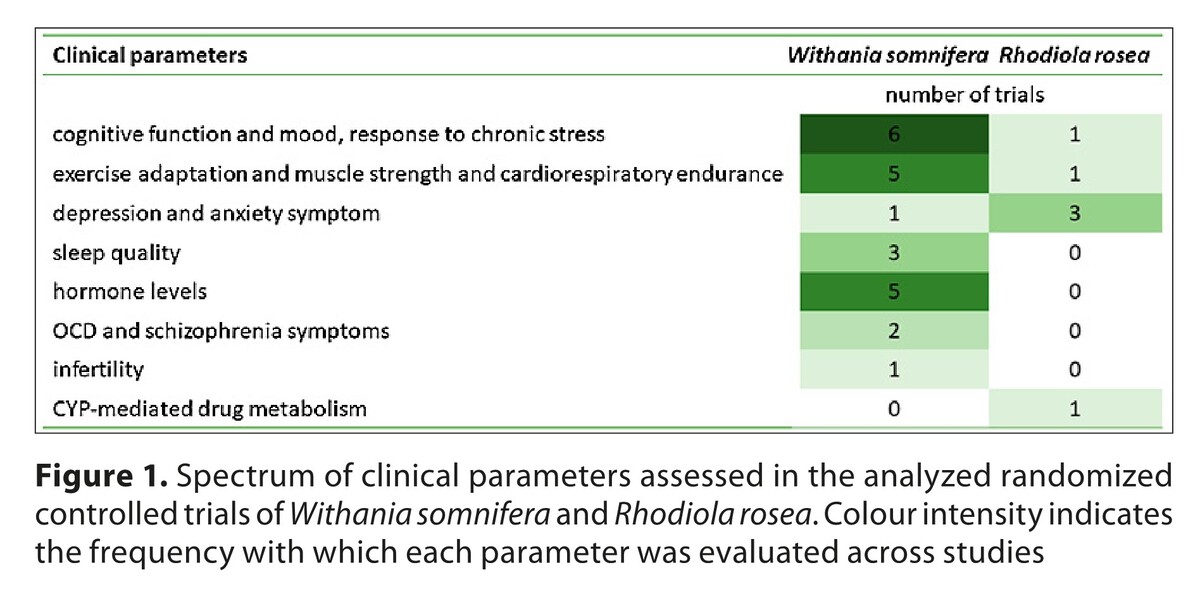
The analyzed trials involved 10–590 participants, aged 18–75 years, both healthy individuals and patients with stress-related or functional disorders. Interventions included standardized extracts at daily doses of 120–1000 mg for W. somnifera and 290–1500 mg for R. rosea, with supplementation lasting 3–16 weeks. Reported benefits included reduction of stress and anxiety, alleviation of depressive symptoms, improved sleep quality, enhancement of cognitive functions, increased muscle strength and recovery, and favourable hormonal changes. Methodological heterogeneity, short intervention periods, and small sample sizes remain limitations.
Summary:
Evidence from RCTs confirms that W. somnifera and R. rosea exert multi-dimensional adaptogenic effects, improving psychophysical health and supporting stress resilience. Their mechanisms involve regulation of the hypothalamic-pituitary-adrenal axis, neurotransmission, immune and hormonal pathways. Further long-term, high-quality clinical trials, supplemented with molecular and systemic approaches, are required to consolidate their role in integrative, evidence-based medicine.
Adaptogens are plant-derived substances that enhance the body’s resilience to physical and psychological stress, with Withania somnifera and Rhodiola rosea being among the most studied representatives. The aim of this review is to evaluate the adaptogenic effects of W. somnifera and R. rosea based on randomized controlled trials (RCTs).
Review methods:
A systematic literature search was performed using the key words: ‘ashwagandha’, ‘Withania somnifera’, ‘Rhodiola rosea’, and ‘plant adaptogen’. Twenty-four studies met the inclusion criteria – 19 on W. somnifera and 5 on R. rosea.
Brief description of the state of knowledge:
The analyzed trials involved 10–590 participants, aged 18–75 years, both healthy individuals and patients with stress-related or functional disorders. Interventions included standardized extracts at daily doses of 120–1000 mg for W. somnifera and 290–1500 mg for R. rosea, with supplementation lasting 3–16 weeks. Reported benefits included reduction of stress and anxiety, alleviation of depressive symptoms, improved sleep quality, enhancement of cognitive functions, increased muscle strength and recovery, and favourable hormonal changes. Methodological heterogeneity, short intervention periods, and small sample sizes remain limitations.
Summary:
Evidence from RCTs confirms that W. somnifera and R. rosea exert multi-dimensional adaptogenic effects, improving psychophysical health and supporting stress resilience. Their mechanisms involve regulation of the hypothalamic-pituitary-adrenal axis, neurotransmission, immune and hormonal pathways. Further long-term, high-quality clinical trials, supplemented with molecular and systemic approaches, are required to consolidate their role in integrative, evidence-based medicine.
REFERENCES (63)
1.
Arumugam V, Vijayakumar V, Balakrishnan AB, et al. Effects of ashwagandha (Withania somnifera) on stress and anxiety: a systematic review and meta-analysis. Explore. 2024;20:103062. doi:10.1016/j.explore.2024.103062.
2.
Panossian AG, Efferth T. Shikov AN, et al. Evolution of the adaptogenic concept from traditional use to medical systems: pharmacology of stress – and aging-related diseases. Med Res Rev. 2021;41:630–703. doi:10.1002/med.21743.
3.
Machín Rubén P, Florido M, Chirino-Godoy R, et al. Adaptogenic botanicals with emphasis on Rhodiola rosea and Withania somnifera. EJMP. 2023;34:20–39. doi:10.9734/ejmp/2023/v34i111168.
4.
Todorova V, Ivanov K, Delattre C, et al. Plant adaptogens-history and future perspectives. Nutrients. 2021;20:2861. doi:10.3390/nu13082861.
5.
Liu XX, Chen CY, Li L, et al. Bibliometric study of adaptogens in dermatology: pharmacophylogeny, phytochemistry, and pharmacological mechanisms. Drug Des Devel Ther. 2023;6:341–361. doi:10.2147/DDDT.S395256.
6.
Mikulska P, Malinowska M, Ignacyk M, et al. Ashwagandha (Withania somnifera) – current research on the health-promoting activities: a narrative review. Pharmaceutics. 2023;15:1057. doi:10.3390/pharmaceutics15041057.
7.
Tóth-Mészáros A, Garmaa G, Hegyi P, et al. The effect of adaptogenic plants on stress: a systematic review and meta-analysis. JFF. 2023;108:105695. https://doi.org/10.1016/j.jff....
8.
Kim H, Choi H, Han K, et al. Ashwagandha (Withania somnifera (L.) dunal) root extract ontaining withanolide a alleviates depression-like behavior in mice by enhancing the brain-derived neurotrophic factor pathway under unexpected chronic mild stress. J Ethnopharmacol. 2025;340:119224. doi:10.1016/j.jep.2024.119224.
9.
Uthirapathy S, Tahir TF. Withania somnifera: correlation of phytoconstituents with hypolipidemic and cardioprotective activities. ARO (Koya). 2021;9:15–21. https://doi.org/10.14500/aro.1...
10.
Udayakumar R, Kasthurirengan S, Mariashibu TS, et al. Hypoglycaemic and hypolipidaemic effects of Withania somnifera root and leaf extracts on alloxan-Induced diabetic rats. Int J Mol Sci. 2009;10:2367–2382. doi:10.3390/ijms10052367.
11.
Owais M, Sharad KS, Shehbaz A, et al. Antibacterial efficacy of Withania somnifera (ashwagandha) an indigenous medicinal plant against experimental murine salmonellosis. Phytomedicine. 2005;12:229–235. doi.org/10.1016/j.phymed.2003.07.012.
12.
Palliyaguru DL, Singh SV, Kensler TW. Withania somnifera: from prevention to treatment of cancer. Mol Nutr Food Res. 2016;60:1342–1353. doi:10.1002/mnfr.201500756.
13.
KrishnaRaju AV, Somepalli V, Thanawala S, et al. Efficacy and anti-inflammatory activity of ashwagandha sustained-release formulation on depression and anxiety induced by chronic unpredictable stress: in vivo and in vitro studies. J Exp Pharmacol. 2023;25:291–305. doi:10.2147/JEP.S407906.
14.
Wiciński M, Fajkiel-Madajczyk A, Kurant Z, et al. Ashwagandha’s multifaceted effects on human health: impact on vascular endothelium, inflammation, lipid metabolism, and cardiovascular outcomes-a review. Nutrients. 2024;31:2481. doi:10.3390/nu16152481.
15.
Wróbel-Biedrawa D, Podolak I. Anti-neuroinflammatory effects of adaptogens: a mini-review. Molecules. 2024;15:866. doi.org/10.3390/molecules29040866.
16.
Sprengel M, Laskowski R, Jost Z. Withania somnifera (ashwagandha) supplementation: a review of its mechanisms, health benefits, and role in sports performance. Nutr Metab (Lond). 2025;5:9. doi:10.1186/s12986-025-00902-7.
17.
Della Porta M, Maier JA, Cazzola R. Effects of Withania somnifera on cortisol levels in stressed human subjects: a systematic review. Nutrients. 2023;5:5015. doi:10.3390/nu15245015.
18.
Alanazi HH, Elfaki E. The immunomodulatory role of Withania somnifera (L.) dunal in inflammatory diseases. Front Pharmacol. 2023;22:1084757. doi:10.3389/fphar.2023.1084757.
19.
Li Y, Cai M, Mao GX, et al. Preclinical evidence and possible mechanisms of Rhodiola rosea L. and its components for ischemic stroke: a systematic review and meta-analysis. Front Pharmacol. 2021;5:736198. doi:10.3389/fphar.2021.736198.
20.
Lu Y, Deng B, Xu L, et al. Effects of Rhodiola rosea supplementation on exercise and sport: a systematic review. Front Nutr. 2022;7:856287. doi:10.3389/fnut.2022.928909.
21.
Bernatoniene J, Jakstas V, Kopustinskiene DM. Phenolic compounds of Rhodiola rosea L. as the potential alternative therapy in the treatment of chronic diseases. Int J Mol Sci. 2023;31:12293. doi:10.3390/ijms241512293.
22.
Tinsley GM, Jagim AR, Potter GDM, et al. Rhodiola rosea as an adaptogen to enhance exercise performance: a review of the literature. Br J Nutr. 2024;14:461–473. doi:10.1017/S0007114523001988.
23.
Xu W, Yang T, Zhang J, et al. Rhodiola rosea: a review in the context of PPPM approach. EPMA J. 2024;27:233–259. doi:10.1007/s13167-024-00367-3.
24.
Wang S, Feng Y, Zheng L, et al. Rosavin: research advances in extraction and synthesis, pharmacological activities and therapeutic effects on diseases of the characteristic active ingredients of Rhodiola rosea L. Molecules. 2023;3:7412. doi:10.3390/molecules28217412.
25.
Tinsley GM, Jagim AR, Potter GDM, et al. Rhodiola rosea as an adaptogen to enhance exercise performance: a review of the literature. Br J Nutr. 2024;14:461–47. doi:10.1017/S0007114523001988.
26.
Li Y, Pham V, Bui M, et al. Rhodiola rosea L.: an herb with anti-stress, anti-aging, and immunostimulating properties for cancer chemoprevention. Curr Pharmacol Rep. 2017;3:384–395. doi:10.1007/s40495-017-0106-1.
27.
Bernatoniene J, Jakstas V, Kopustinskiene DM. Phenolic compounds of Rhodiola rosea L. as the potential alternative therapy in the treatment of chronic diseases. Int J Mol Sci. 2023;31:1229. doi:10.3390/ijms241512293.
28.
Elgendy SA, Soliman MM, Ghamry HI, et al. Exploration of tilmicosin cardiotoxicity in rats and the protecting role of the Rhodiola rosea extract: potential roles of cytokines, antioxidant, apoptotic, and anti-fibrotic pathways. Toxics. 2023;13:857. https://doi.org/10.3390/toxics...
29.
Berger VW, Bour LJ, Carter K, et al. Randomization innovative design scientific working group. A roadmap to using randomization in clinical trials. BMC Med Res Methodol. 2021;16:168. doi:10.1186/s12874-021-01303-z.
30.
Spieth PM, Kubasch AS, Penzlin AI, et al. Randomized controlled trials – a matter of design. Neuropsychiatr Dis Treat. 2016;10:1341–1349. doi:10.2147/NDT.S101938.
31.
Hariton E, Locascio JJ. Randomised controlled trials – the gold standard for effectiveness research: study design: randomised controlled trials. BJOG. 2018;125:1716. doi:10.1111/1471-0528.15199.
32.
Zabor EC, Kaizer AM, Hobbs BP. Randomized controlled trials. Chest. 2020;158:S79–S87. doi:10.1016/j.chest.2020.03.013.
33.
Fernainy P, Cohen AA, Murray E, et al. Rethinking the pros and cons of randomized controlled trials and observational studies in the era of big data and advanced methods: a panel discussion. BMC Proc. 2024;18:1. https://doi.org/10.1186/s12919...
34.
Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews BMJ. 2021;372:71. https://doi.org/10.1136/bmj.n7....
35.
Coope OC, Reales Salguero A, Spurr T, et al. Effects of root extract of ashwagandha (Withania somnifera) on perception of recovery and muscle strength in female athletes. Eur J Sport Sci. 2025;25:e12265. doi:10.1002/ejsc.12265.
36.
Verma N, Gupta SK, Patil S, et al. Effects of ashwagandha (Withania somnifera) standardized root extract on physical endurance and VO2max in healthy adults performing resistance training: an eight-week, prospective, randomized, double-blind, placebo-controlled study. F1000Res. 2024;8:335. doi:10.12688/f1000research.130932.2.
37.
Wankhede S, Langade D, Joshi K, et al. Examining the effect of Withania somnifera supplementation on muscle strength and recovery: a randomized controlled trial. J Int Soc Sports Nutr. 2015;25:43. doi:10.1186/s12970-015-0104-9.
38.
Choudhary D, Bhattacharyya S, Joshi K. Body weight management in adults under chronic stress through treatment with ashwagandha root extract: a double-blind, randomized, placebo-controlled trial. J Evid Based Complementary Altern Med. 2017;22:96–106. doi:10.1177/2156587216641830.
39.
Choudhary D, Bhattacharyya S, Bose S. Efficacy and safety of ashwagandha (Withania somnifera (L.) dunal) root extract in improving memory and cognitive functions. J Diet Suppl. 2017;2:599–612. doi:10.1080/19390211.2017.1284970.
40.
Langade D, Thakare V, Kanchi S, et al. Clinical evaluation of the pharmacological impact of ashwagandha root extract on sleep in healthy volunteers and insomnia patients: a double-blind, randomized, parallel-group, placebo-controlled study. J Ethnopharmacol. 2021;264:113276. doi:10.1016/j.jep.2020.113276.
41.
Gopal S, Ajgaonkar A, Kanchi P, et al. Effect of an ashwagandha (Withania somnifera) root extract on climacteric symptoms in women during perimenopause: a randomized, double-blind, placebo-controlled study. J Obstet Gynaecol Res. 2021;47:4414–4425. doi:10.1111/jog.15030.
42.
Tiwari S, Gupta SK, Pathak AK. A double-blind, randomized, placebo-controlled trial on the effect of ashwagandha (Withania somnifera dunal.) root extract in improving cardiorespiratory endurance and recovery in healthy athletic adults. J Ethnopharmacol. 2021;23:113929. doi:10.1016/j.jep.2021.113929.
43.
Ziegenfuss TN, Kedia AW, Sandrock JE, et al. Effects of an aqueous extract of Withania somnifera on strength training adaptations and recovery: the STAR trial. Nutrients. 2018;20:1807. doi:10.3390/nu10111807.
44.
Pandit S, Srivastav AK, Sur TK, et al. Effects of Withania somnifera extract in chronically stressed adults: a randomized controlled trial. Nutrient. 2024;26:1293. doi:10.3390/nu16091293.
45.
Gannon JM, Brar J, Rai A, et al. Effects of a standardized extract of Withania somnifera (ashwagandha) on depression and anxiety symptoms in persons with schizophrenia participating in a randomized, placebo-controlled clinical trial. Ann Clin Psychiatry. 2019;31:123–129.
46.
Lopresti AL, Smith SJ, Malvi H, et al. An investigation into the stress-relieving and pharmacological actions of an ashwagandha (Withania somnifera) extract: a randomized, double-blind, placebo-controlled study. Medicine (Baltimore). 2019;98:e17186. doi:10.1097/MD.0000000000017186.
47.
Deshpande A, Irani N, Balkrishnan R, et al. A randomized, double blind, placebo controlled study to evaluate the effects of ashwagandha (Withania somnifera) extract on sleep quality in healthy adults. Sleep Med. 2020;72:28–36. doi:10.1016/j.sleep.2020.03.012.
48.
Lopresti AL, Drummond PD, Smith SJ. A randomized, double-blind, placebo-controlled, crossover study examining the hormonal and vitality effects of ashwagandha (Withania somnifera) in aging, overweight males. Am J Mens Health. 2019;13:1557988319835985. doi:10.1177/1557988319835985.
49.
Smith SJ, Lopresti AL, Fairchild TJ. Exploring the efficacy and safety of a novel standardized ashwagandha (Withania somnifera) root extract (Witholytin®) in adults experiencing high stress and fatigue in a randomized, double-blind, placebo-controlled trial. J Psychopharmacol. 2023;37:1091–1104. doi:10.1177/02698811231200023.
50.
Leonard M, Dickerson B, Estes L, et al. Acute and repeated ashwagandha supplementation improves markers of cognitive function and mood. Nutrients. 2024;8:1813. doi:10.3390/nu16121813.
51.
Jahanbakhsh SP, Manteghi AA, Emami SA, et al. Evaluation of the efficacy of Withania somnifera (ashwagandha) root extract in patients with obsessive-compulsive disorder: a randomized double-blind placebo-controlled trial. Complement Ther Med. 2016;27:25–29. doi:10.1016/j.ctim.2016.03.018.
52.
Deshpande A, Irani N, Balakrishnan R. Study protocol and rationale for a prospective, randomized, double-blind, placebo-controlled study to evaluate the effects of ashwagandha (Withania somnifera) extract onnonrestorative sleep. Medicine (Baltimore). 2018;97:e11299. doi:10.1097/MD.0000000000011299.
53.
Nasimi Doost Azgomi R, Nazemiyeh H, Sadeghi Bazargani H, et al. Comparative evaluation of the effects of Withania somnifera with pentoxifylline on the sperm parameters in idiopathic male infertility: a triple-blind randomised clinical trial. Andrologia. 2018;50:e13041. doi:10.1111/and.13041.
54.
Williams TD, Langley HN, Roberson CC, et al. Effects of short-term golden root extract (Rhodiola rosea) supplementation on resistance exercise performance. Int J Environ Res Public Health. 2021;29:6953. doi:10.3390/ijerph18136953.
55.
Thu OK, Spigset O, Nilsen OG, et al. Effect of commercial Rhodiola rosea on CYP enzyme activity in humans. Eur J Clin Pharmacol. 2016;72:295–300. doi:10.1007/s00228-015-1988-7.
56.
Cropley M, Banks AP, Boyle J. The effects of Rhodiola rosea L. Extract on anxiety, stress, ccognition and other mood symptoms. Phytother Res. 2015;29:1934–1939. doi:10.1002/ptr.5486.
57.
Mao JJ, Xie SX, Zee J, et al. Rhodiola rosea versus sertraline for major depressive disorder: a randomized placebo-controlled trial. Phytomedicine. 2015;15:394–399. doi:10.1016/j.phymed.2015.01.010.
58.
Gao L. Wu C, Liao Y, et al. Antidepressants effects of Rhodiola capsule combined with sertraline for major depressive disorder: a randomized double-blind placebo-controlled clinical trial. J Affect Disord. 2020;15:99–103. doi:10.1016/j.jad.2020.01.065.
59.
Thompson, E. Hamilton rating scale for anxiety (HAM-A). Occup Med. 2015;65:601. doi:10.1093/occmed/kqv054.
60.
Harris KM, Gaffey AE, Schwartz J, et al. The perceived stress scale as a measure of stress: decomposing score variance in longitudinal behavioral medicine studies. Ann Behav Med. 2023;13:846–854. doi:10.1093/abm/kaad015.
61.
Vorland CJ, Brown AW, Dawson JA, et al. Errors in the implementation, analysis, and reporting of randomization within obesity and nutrition research: a guide to their avoidance. Int J Obes. 2021;45:2335–2346. https://doi.org/10.1038/s41366...
62.
Lyman GH, Desai A, Leyfman Y, et al. Opportunities and challenges of observational studies and randomized controlled trials for evaluating the therapeutic efficacy of COVID-19 convalescent plasma. Cancer Invest. 2021;39:449–456. doi:10.1080/07357907.2021.1942127.
63.
Frieden TR. Evidence for health decision making – beyond randomized, controlled trials. N Engl J Med. 2017;3:465–475. doi:10.1056/NEJMra1614394.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.