Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
RESEARCH PAPER
Analysis of novel serum markers of fibrosis and angiogenesis in patients with alcoholic liver
cirrhosis
1
Department of Internal Medicine, Medical University, Lublin, Poland
2
Department of Experimental Haematooncology, Medical University, Lublin, Poland
3
Department of Medical Chemistry, Medical University, Lublin, Poland
4
Department of Family Medicine and Community Nursing, Medical University, Lublin, Poland
5
Department of Internal Diseases and Hypertension, Institute of Rural Health, Lublin, Poland
Corresponding author
Ann Agric Environ Med. 2020;27(4):568-573
KEYWORDS
alcoholic liver cirrhosisangiopoietin-like peptide 4 (ANGPTL-4)asialoglycoprotein receptor 1 (ASGP-R1)S100 calcium-binding protein A8 (S100A8)hyaluronic acid (Hyal)collagen IV (Coll IV)
TOPICS
ABSTRACT
Introduction:
Alcohol consumption causes acute and chronic liver injury. The clinical forms of alcohol liver disease (ALD) include steatosis, hepatitis, cirrhosis, and hepatocellular carcinoma (HCC) associated with liver cirrhosis.
Objective:
The aim of the study was to determine the levels of novel markers of fibrogenesis and angiogenesis in patients with alcoholic liver cirrhosis. Serum levels of angiopoietin-like peptide 4 (ANGPTL-4), asialoglycoprotein receptor 1 (ASGP-R1), and S100 calcium-binding protein A8 (S100A8) were assessed. Levels of hyaluronic acid (Hyal) and collagen IV (Coll IV) werealso determined at various stages of alcoholic liver cirrhosis.
Material and methods:
The study group consisted of 72 patients with alcoholic liver cirrhosis, while the control group included 22 healthy subjects without a history of alcohol abuse. The degree of liver cirrhosis was evaluated according to the Pugh-Child criteria (Pugh-Child score). Based on thse scores, patients were assigned to one of three groups: Pugh-Child (P-Ch) A – 21 with stage A, P-Ch B – 23 with stage B and P-Ch C – 28 with stage C liver cirrhosis. Serum levels of markers were determined using ELISA.
Results:
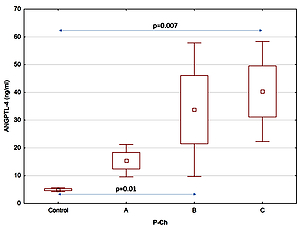
The study findings demonstrated higher levels of ANGPTL-4, ASGP-R1, S100A, hyaluronic acid and serum collagen IV in the group of patients with alcoholic liver cirrhosis, compared to the control group. Furthermore, their levels increased with the progression of alcoholic liver cirrhosis.
Conclusions:
The biomarkers analysed in the study may be useful for diagnosis and prognosis in patients with alcoholic liver cirrhosis.
Alcohol consumption causes acute and chronic liver injury. The clinical forms of alcohol liver disease (ALD) include steatosis, hepatitis, cirrhosis, and hepatocellular carcinoma (HCC) associated with liver cirrhosis.
Objective:
The aim of the study was to determine the levels of novel markers of fibrogenesis and angiogenesis in patients with alcoholic liver cirrhosis. Serum levels of angiopoietin-like peptide 4 (ANGPTL-4), asialoglycoprotein receptor 1 (ASGP-R1), and S100 calcium-binding protein A8 (S100A8) were assessed. Levels of hyaluronic acid (Hyal) and collagen IV (Coll IV) werealso determined at various stages of alcoholic liver cirrhosis.
Material and methods:
The study group consisted of 72 patients with alcoholic liver cirrhosis, while the control group included 22 healthy subjects without a history of alcohol abuse. The degree of liver cirrhosis was evaluated according to the Pugh-Child criteria (Pugh-Child score). Based on thse scores, patients were assigned to one of three groups: Pugh-Child (P-Ch) A – 21 with stage A, P-Ch B – 23 with stage B and P-Ch C – 28 with stage C liver cirrhosis. Serum levels of markers were determined using ELISA.
Results:
The study findings demonstrated higher levels of ANGPTL-4, ASGP-R1, S100A, hyaluronic acid and serum collagen IV in the group of patients with alcoholic liver cirrhosis, compared to the control group. Furthermore, their levels increased with the progression of alcoholic liver cirrhosis.
Conclusions:
The biomarkers analysed in the study may be useful for diagnosis and prognosis in patients with alcoholic liver cirrhosis.
ACKNOWLEDGEMENTS
This study has been performed at Medical University of Lublin, Poland, and it was funded by a Grant from the Medical University of Lublin (DS 507/2013–2015). The authors thank Anna Misiuna, who provided medical writing services on behalf of Medical University of Lublin, Poland.
Prystupa A, Kiciński P, Luchowska-Kocot D, Nowicki G, Dzida G, Myśliński W, Zakrzewski M, Mosiewicz J, Panasiuk L. Analysis of novel
serum markers of fibrosis and angiogenesis in patients with alcoholic liver cirrhosis. Ann Agric Environ Med. 2020; 27(4): 568–573.
doi: 10.26444/aaem/127621
REFERENCES (27)
1.
Dunn W, Shah VH. Pathogenesis of Alcoholic Liver Disease. Clin Liver Dis. 2016; 20: 445–456.
2.
Osna NA, Donohue Jr. TM, Kharbanda KK. Alcoholic Liver Disease: Pathogenesis and Current Management. Alcohol Res. 2017; 38: 147–161.
3.
Moreno C, Mueller S, Szabo GJ. Non-invasive diagnosis and biomarkers in alcohol-related liver disease. J Hepatol. 2019 F; 70: 273–283.
4.
Prystupa A, Kiciński P, Sak J, Boguszewska-Czubara A, Toruń-Jurkowska A, Załuska W. Proinflammatory Cytokines (IL-1?, IL-6) and Hepatocyte Growth Factor in Patients with Alcoholic Liver Cirrhosis. Gastroenterol Res Pract. 2015; 2015: 532615.
5.
Elpek GÖ. Angiogenesis and liver fibrosis. World J Hepatol. 2015; 7: 377–391.
6.
Friedman SL. Molecular regulation of hepatic fibrosis, an integrated cellular response to tissue injury. J Biol Chem. 2000; 275: 2247–2250.
7.
Tsutsumi M, Urashima S, Matsuda Y, Takase S, Takada A. Changes in type IV collagen content in livers of patients with alcoholic liver disease. Hepatology. 1993; 17: 820–827.
8.
Gressner AM, Haarmann R. Hyaluronic acid synthesis and secretion by rat liver fatstoring cells (perisinusoidal lipocytes) in culture. Biochem Biophys Res Commun. 1988; 151: 222–229.
9.
Nacken W, Roth J, Sorg C, Kerkhoff C. S100A9/S100A8: Myeloid representatives of the S100 protein family as prominent players in innate immunity. Microsc Res Tech. 2003; 60: 569–580.
10.
De Ponti A, Wiechert L, Stojanovic A, Longerich T, Marhenke S, Hogg N, et al. Chronic liver inflammation and hepatocellular carcinogenesis are independent of S100A9. Int J Cancer. 2015; 136: 2458–2463.
11.
Wang S, R. Song R, Wang Z, Jing Z, Wang S, Ma J. S100A8/A9 in Inflammation. Front Immunol. 2018; 9: 1298.
12.
Serhal R, Hilal G, Boutros G, Sidaoui J, Wardi L, Ezzeddine S, et al. Nonalcoholic Steatohepatitis: Involvement of the Telomerase and Proinflammatory Mediators. Biomed Res Int. 2015; 2015: 850246.
13.
De Ponti A, Wiechert L, Schneller D, Pusterla T, Longerich T, Hogg N, et al. A pro-tumorigenic function of S100A8/A9 in carcinogen-induced hepatocellular carcinoma. Cancer Lett. 2015; 369: 396–404.
14.
Hoekstra LT, de Graaf W, Nibourg GAA, Heger M, Bennink RJ, Stieger B, van Gulik TM. Physiological and biochemical basis of clinical liver function tests: a review. Ann Surg. 2013; 257: 27–36.
15.
Nakaya R, Kohgo Y, Mogi Y, Nakajima M, Kato J, Niitsu Y. Regulation of asialoglycoprotein receptor synthesis by inflammation-related cytokines in HepG2 cells. J Gastroenterol. 1994; 29: 24–30.
16.
Zhang D, Guo Z, Zhang P, Li Y, Su X, You L, et al. Simplified quantification method for in vivo SPECT/CT imaging of asialoglycoprotein receptor with (99m)Tc-p(VLA-co-VNI) to assess and stage hepatic fibrosis in mice. Sci Rep. 2016; 6: 25377.
17.
Witzigmann D, Quagliata L, Schenk SH, Quintavalle C, Terracciano LM, Huwyler J. Variable asialoglycoprotein receptor 1 expression in liver disease: Implications for therapeutic intervention. Hepatol Res. 2016; 46: 686–696.
18.
Morinaga J, Zhao J, Endo M, Kadomatsu T, Miyata K, Sugizaki T, et al. Association of circulating ANGPTL 3, 4, and 8 levels with medical status in a population undergoing routine medical checkups: A cross-sectional study. PLoS One 2018; 13: e0193731.
19.
Barja-Fernández S, Folgueira C, Castelao C, Pena-León V, González-Saenz P, Vázquez-Cobela R, et al. ANGPTL-4 is Associated with Obesity and Lipid Profile in Children and Adolescents. Nutrients 2019; 11: 1340.
20.
Gealekman O, Guseva N, Hartigan C, Apotheker S, Gorgoglione M, Gurav K, et. al. Depot-specific differences and insufficient subcutaneous adipose tissue angiogenesis in human obesity. Circulation. 2011; 123: 186–194.
21.
Morinaga J, Zhao J, Endo M, Kadomatsu T, Miyata K, Sugizaki T, et al. Association of circulating ANGPTL 3, 4, and 8 levels with medical status in a population undergoing routine medical checkups: A cross-sectional study. PloS One 2018; 13: e019373.
22.
Altun Ö, Dikker O, Arman Y, Ugurlukisi B, Kutlu O, Cil EO, et al. Serum Angiopoietin-like peptide 4 levels in patients with hepatic steatosis. Cytokine 2018; 111: 496–499.
23.
Dikker O, Çetin Dag N, Şahin M, Türkkan E, Dag H. The association of angiopoietin-like peptide 4 levels with obesity and hepatosteatosis in adolescents. Cytokine 2020; 125: 154802.
24.
Lu B, Moser A, Shigenaga JK, Grunfeld C, Feingold KR. The acute phase response stimulates the expression of angiopoietin like protein 4. Biochem Biophys Res Commun. 2010; 391: 1737–1741.
25.
El-Shal AS, Zidan HE, Rashad NM, Wadea FM. Angiopoietin-like protein 3 and 4 expression 4 and their serum levels in hepatocellular carcinoma. Cytokine 2017; 96: 75–86.
26.
Ng K TP, Xu A, Cheng Q, Guo DY, Xue-Hui Lim Z, Kin_wai Sun Ch, et al. Clinical relevance and therapeutic potential of angiopoietin-like protein 4 in hepatocellular carcinoma. Mol Cancer. 2014; 13: 196.
27.
Li H, Ge C, Zhao F, Yan M, Hu Ch, Jia D, et al. Hypoxia-inducible factor 1 alpha–activated angiopoietin-like protein 4 contributes to tumor metastasis via vascular cell adhesion molecule-1/integrin ß1 signaling in human hepatocellular carcinoma. Hepatology 2011; 54: 910–919.
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.