Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
RESEARCH PAPER
2,4-dinitrophenol enhances cisplatin and etoposide cytotoxic activity on prostate cancer cell line
1
Independent Medical Biology Unit, Medical University, Lublin, Poland
2
Department of Biochemistry and Molecular Biology, Medical University, Lublin, Poland
3
Department of Human Anatomy, Medical University, Lublin, Poland
4
Department of Toxicology, Medical University, Lublin, Poland
Corresponding author
Ann Agric Environ Med. 2024;31(1):37-46
KEYWORDS
TOPICS
ABSTRACT
Introduction and objective:
Including additional compounds that disturb the energy metabolism of cancer cells in advanced cancer therapy regimens may be an approach to overcome the problem of drug resistance and the therapeutic effectiveness of classic chemotherapeutics. One of the compounds that decouple oxidative phosphorylation, and thus alter the activity of energy-producing pathways, is 2,4-DNP (2,4- dinitrophenol).
Objective:
The aim of the study was to assess the ability of the 2,4-DNP to sensitize prostate cancer cells to the action of cisplatin and etoposide, or to intensify their action.
Material and methods:
The research was carried out on three prostate cancer cell lines (LNCaP, PC-3, DU-145. To assess the effect of cisplatin or etoposide with 2,4-DNP on prostate cancer cells, MTT assay, analysis of the cell cycle and apoptosis detection was performed. Oxidative stress was investigated by CellRox fluorescence staining and expression of genes related to antioxidant defence. In addition, analysis was conducted of the expression of genes related to cell cycle inhibition, transporters associated with multi-drug resistance and DNA repair.
Results:
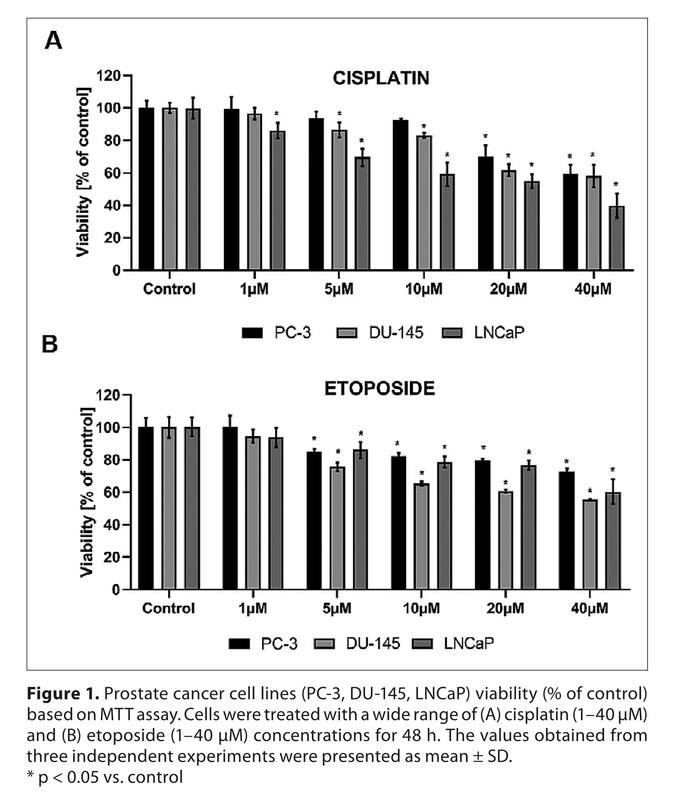
The study showed that the simultaneous incubation of 2,4-DNP with cisplatin or etoposide enhances the cytotoxic effect of the chemotherapeutic agent only in LNCaP cells (oxidative phenotype).
Conclusions:
The enhanced cytotoxic effect of chemotherapeutics by 2,4-DNP may be the result of disturbed redox balance, reduced ability of cells to repair DNA, and the oxidative metabolic phenotype of prostate cancer cells.
Including additional compounds that disturb the energy metabolism of cancer cells in advanced cancer therapy regimens may be an approach to overcome the problem of drug resistance and the therapeutic effectiveness of classic chemotherapeutics. One of the compounds that decouple oxidative phosphorylation, and thus alter the activity of energy-producing pathways, is 2,4-DNP (2,4- dinitrophenol).
Objective:
The aim of the study was to assess the ability of the 2,4-DNP to sensitize prostate cancer cells to the action of cisplatin and etoposide, or to intensify their action.
Material and methods:
The research was carried out on three prostate cancer cell lines (LNCaP, PC-3, DU-145. To assess the effect of cisplatin or etoposide with 2,4-DNP on prostate cancer cells, MTT assay, analysis of the cell cycle and apoptosis detection was performed. Oxidative stress was investigated by CellRox fluorescence staining and expression of genes related to antioxidant defence. In addition, analysis was conducted of the expression of genes related to cell cycle inhibition, transporters associated with multi-drug resistance and DNA repair.
Results:
The study showed that the simultaneous incubation of 2,4-DNP with cisplatin or etoposide enhances the cytotoxic effect of the chemotherapeutic agent only in LNCaP cells (oxidative phenotype).
Conclusions:
The enhanced cytotoxic effect of chemotherapeutics by 2,4-DNP may be the result of disturbed redox balance, reduced ability of cells to repair DNA, and the oxidative metabolic phenotype of prostate cancer cells.
REFERENCES (45)
2.
Amoedo ND, Valencia JP, Rodrigues MF, Galina A, Rumjanek FD. How does the metabolism of tumour cells differ from that of normal cells. Bioscience Reports. 2013;33(6). doi:10.1042/BSR20130066.
3.
Kalyanaraman B. Teaching the basics of cancer metabolism: Developing antitumour strategies by exploiting the differences between normal and cancer cell metabolism. Redox Biol. 2017;12:833–842. doi:10.1016/j.redox.2017.04.018.
4.
Dagogo-Jack I, Shaw AT. Tumour heterogeneity and resistance to cancer therapies. Nat Rev Clin Oncol. 2018;15(2):81–94. doi:10.1038/nrclinonc.2017.166.
5.
Parsons BL. Multiclonal tumour origin: Evidence and implications. Mutat Res Rev Mutat Res. 2018;777:1–18. doi:10.1016/j.mrrev.2018.05.001.
6.
Baghban R, Roshangar L, Jahanban-Esfahlan R, et al. Tumour microenvironment complexity and therapeutic implications at a glance. Cell Communication and Signaling. 2020;18(1):59. doi:10.1186/s12964-020-0530-4.
7.
Rui L. New Antidiabetes Agent Targeting Both Mitochondrial Uncoupling and Pyruvate Catabolism: Two Birds With One Stone. Diabetes. 2019;68(12):2195–2196. doi:10.2337/dbi19-0024.
8.
Geisler JG. 2,4 Dinitrophenol as Medicine. Cells. 2019;8(3):E280. doi:10.3390/cells8030280.
9.
Grundlingh J, Dargan PI, El-Zanfaly M, Wood DM. 2,4-Dinitrophenol (DNP): A Weight Loss Agent with Significant Acute Toxicity and Risk of Death. J Med Toxicol. 2011;7(3):205–212. doi:10.1007/s13181-011-0162-6.
10.
Han YH, Kim SW, Kim SH, Kim SZ, Park WH. 2,4-dinitrophenol induces G1 phase arrest and apoptosis in human pulmonary adenocarcinoma Calu-6 cells. Toxicol In Vitro. 2008;22(3):659–670. doi:10.1016/j.tiv.2007.12.005.
11.
Berghmans T, Durieux V, Holbrechts S, et al. Systemic treatments for thymoma and thymic carcinoma: A systematic review. Lung Cancer. 2018;126:25–31. doi:10.1016/j.lungcan.2018.10.018.
12.
Shi H, Guo N, Zhao Z, et al. Comparison of the second-line treatments for patients with small cell lung cancer sensitive to previous platinum-based chemotherapy: A systematic review and Bayesian network analysis. Front Oncol. 2023;13:1154685. doi:10.3389/fonc.2023.1154685.
13.
Funt SA, McHugh DJ, Tsai S, et al. Four Cycles of Etoposide plus Cisplatin for Patients with Good-Risk Advanced Germ Cell Tumours. Oncologist. 2021;26(6):483–491. doi:10.1002/onco.13719.
14.
Dasari S, Tchounwou PB. Cisplatin in cancer therapy: molecular mechanisms of action. Eur J Pharmacol. 2014;740:364–378. doi:10.1016/j.ejphar.2014.07.025.
15.
Montecucco A, Zanetta F, Biamonti G. Molecular mechanisms of etoposide. EXCLI J. 2015;14:95–108. doi:10.17179/excli2015-561.
16.
Shin HJ, Kwon HK, Lee JH, Anwar MA, Choi S. Etoposide induced cytotoxicity mediated by ROS and ERK in human kidney proximal tubule cells. Sci Rep. 2016;6(1):34064. doi:10.1038/srep34064.
17.
Chaiswing L, St Clair WH, St Clair DK. Redox Paradox: A Novel Approach to Therapeutics-Resistant Cancer. Antioxid Redox Signal. 2018;29(13):1237–1272. doi:10.1089/ars.2017.7485.
18.
Li L ya, Guan Y di, Chen X sha, Yang J ming, Cheng Y. DNA Repair Pathways in Cancer Therapy and Resistance. Front Pharmacol. 2021;11:629266. doi:10.3389/fphar.2020.629266.
19.
Zheng Y, Ma L, Sun Q. Clinically-Relevant ABC Transporter for Anti-Cancer Drug Resistance. Frontiers in Pharmacology. 2021;12. Accessed March 14, 2023. https://www.frontiersin.org/ar....
20.
Higgins LH, Withers HG, Garbens A, et al. Hypoxia and the metabolic phenotype of prostate cancer cells. Biochim Biophys Acta. 2009;1787(12):1433–1443. doi:10.1016/j.bbabio.2009.06.003.
21.
Adamczuk GM, Humeniuk E, Adamczuk K, Madej-Czerwonka B, Dudka J. Disruption of mitochondrial function augments the radiosensitivity of prostate cancer cell lines. Ann Agric Environ Med. Published online October 21, 2022. doi:10.26444/aaem/155382.
22.
Adamczuk G, Humeniuk E, Adamczuk K, Grabarska A, Dudka J. 2,4-Dinitrophenol as an Uncoupler Augments the Anthracyclines Toxicity against Prostate Cancer Cells. Molecules. 2022;27(21):7227. doi:10.3390/molecules27217227.
23.
Kuruburu MG, Bovilla VR, Naaz R, Leihang Z, Madhunapantula SV. Variations in the Anticancer Activity of Free and Bound Phenolics of Finger Millet (Eleusine coracana (L) Gaertn; Variety KMR-301) Seeds. Phytomedicine Plus. 2022;2(2):100276. doi:10.1016/j.phyplu.2022.100276.
24.
Allambergenova Z, Kasela M, Adamczuk G, et al. Phytochemical Profile and Biological Activity of the Ethanolic Extract from the Aerial Part of Crocus alatavicus Regel & Semen Growing Wildly in Southern Kazakhstan. Molecules. 2022;27(11):3468. doi:10.3390/molecules27113468.
25.
Maszczyk M, Banach K, Karkoszka M, et al. Chemosensitization of U-87 MG Glioblastoma Cells by Neobavaisoflavone towards Doxorubicin and Etoposide. Int J Mol Sci. 2022;23(10):5621. doi:10.3390/ijms23105621.
26.
Bonnay F, Veloso A, Steinmann V, et al. Oxidative Metabolism Drives Immortalization of Neural Stem Cells during Tumourigenesis. Cell. 2020;182(6):1490–1507.e19. doi:10.1016/j.cell.2020.07.039.
27.
Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162(1):156–159. doi:10.1006/abio.1987.9999.
28.
Rajkumar P, Mathew BS, Das S, et al. Cisplatin Concentrations in Long and Short Duration Infusion: Implications for the Optimal Time of Radiation Delivery. J Clin Diagnostic Res. JCDR. 2016;10(7):XC01. doi:10.7860/JCDR/2016/18181.8126.
29.
Bruni E, Reichle A, Scimeca M, Bonanno E, Ghibelli L. Lowering Etoposide Doses Shifts Cell Demise From Caspase-Dependent to Differentiation and Caspase-3-Independent Apoptosis via DNA Damage Response, Inducing AML Culture Extinction. Front Pharmacol. 2018;9:1307. doi:10.3389/fphar.2018.01307.
30.
Geraldo de Campos E, Fogarty M, Spinosa De Martinis B, Kerr Logan B. Analysis of 2,4-Dinitrophenol in Postmortem Blood and Urine by Gas Chromatography-Mass Spectrometry: Method Development and Validation and Report of Three Fatalities in the United States. J Forensic Sci. 2020;65(1):183–188. doi:10.1111/1556-4029.14154.
31.
Liberti MV, Locasale JW. The Warburg Effect: How Does it Benefit Cancer Cells? Trends Biochem Sci. 2016;41(3):211–218. doi:10.1016/j.tibs.2015.12.001.
32.
Wheaton WW, Weinberg SE, Hamanaka RB, et al. Metformin inhibits mitochondrial complex I of cancer cells to reduce tumourigenesis. Elife. 2014;3:e02242. doi:10.7554/eLife.02242.
33.
Fontaine E. Metformin-Induced Mitochondrial Complex I Inhibition: Facts, Uncertainties, and Consequences. Frontiers in Endocrinology. 2018;9. Accessed March 14, 2023. https://www.frontiersin.org/ar....
34.
Bastian A, Matsuzaki S, Humphries KM, et al. AG311, a small molecule inhibitor of complex I and hypoxia-induced HIF-1? stabilization. Cancer Lett. 2017;388:149–157. doi:10.1016/j.canlet.2016.11.040.
35.
Chen H, Li L, Lu Y, et al. Azoxystrobin Reduces Oral Carcinogenesis by Suppressing Mitochondrial Complex III Activity and Inducing Apoptosis. CMAR. 2020;12:11573–11583. doi:10.2147/CMAR.S280285.
36.
Stephenson ZA, Harvey RF, Pryde KR, et al. Identification of a novel toxicophore in anti-cancer chemotherapeutics that targets mitochondrial respiratory complex I. Elife. 2020;9:e55845. doi:10.7554/eLife.55845.
37.
Nam C, Doi K, Nakayama H. Etoposide induces G2/M arrest and apoptosis in neural progenitor cells via DNA damage and an ATM/p53-related pathway. Histol Histopathol. 2010;25(4):485–493. doi:10.14670/HH-25.485.
38.
Velma V, Dasari SR, Tchounwou PB. Low Doses of Cisplatin Induce Gene Alterations, Cell Cycle Arrest, and Apoptosis in Human Promyelocytic Leukemia Cells. Biomark Insights. 2016;11:113–121. doi:10.4137/BMI.S39445.
39.
Wang P, Cui J, Wen J, Guo Y, Zhang L, Chen X. Cisplatin induces HepG2 cell cycle arrest through targeting specific long noncoding RNAs and the p53 signaling pathway. Oncology Letters. 2016;12(6):4605–4612. doi:10.3892/ol.2016.5288.
40.
Kreis NN, Louwen F, Yuan J. The Multifaceted p21 (Cip1/Waf1/CDKN1A) in Cell Differentiation, Migration and Cancer Therapy. Cancers. 2019;11(9):1220. doi:10.3390/cancers11091220.
41.
Yu W, Chen Y, Dubrulle J, et al. Cisplatin generates oxidative stress which is accompanied by rapid shifts in central carbon metabolism. Sci Rep. 2018;8(1):4306. doi:10.1038/s41598-018-22640-y.
42.
Zalcberg J, Hu XF, Slater A, et al. MRP1 not MDR1 gene expression is the predominant mechanism of acquired multidrug resistance in two prostate carcinoma cell lines. Prostate Cancer Prostatic Dis. 2000;3(2):66–75. doi:10.1038/sj.pcan.4500394.
43.
Schinkel AH, Jonker JW. Mammalian drug efflux transporters of the ATP binding cassette (ABC) family: an overview. Adv Drug Deliv Rev. 2003;55(1):3–29. doi:10.1016/s0169-409x(02)00169-2.
44.
Wang JQ, Wu ZX, Yang Y, et al. ATP-binding cassette (ABC) transporters in cancer: A review of recent updates. J Evidence-Based Med. 2021;14(3):232–256. doi:10.1111/jebm.12434.
45.
Giddings EL, Champagne DP, Wu MH, et al. Mitochondrial ATP fuels ABC transporter-mediated drug efflux in cancer chemoresistance. Nat Commun. 2021;12(1):2804. doi:10.1038/s41467-021-23071-6.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.