Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
RESEARCH PAPER
Pulmonary mycobacteriosis of sitatunga antelope caused by M. avium ssp. hominissuis
1
National Veterinary Research Institute, Puławy, Poland
2
Zoological Garden, Gdańsk, Poland
Corresponding author
Ann Agric Environ Med. 2022;29(2):220-223
KEYWORDS
TOPICS
ABSTRACT
Introduction and objective:
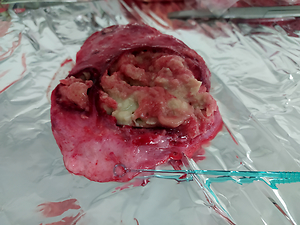
Mycobacteriosis are diseases caused by acid-fast mycobacteria other than M. leprae and tuberculous mycobacteria. Animal mycobacteriosis is often caused by M. avium ssp. hominissuis. Many species of animals are susceptible to infection with this bacterium, even those kept in Zoological Gardens. The aim of the study was to determine the species of bacterium responsible for causing the disease in the tested animals.
Material and methods:
Tissue samples of two male sitatunga antelopes (Tragelaphus spekii) were analyzed. Lymph node and lung samples were subjected to anatomical examination and Ziehl-Neelsen staining. Real-time PCR was performed to confirm or rule out tuberculosis mycobacteria infection. In order to isolate the bacterial strain, tissue samples were inoculated on both solid and liquid media. HainLifescience CM tests, mass spectrometry and New Generation Sequencing were used to determine the mycobacterial species.
Results:
Results showed that atypical mycobacteria are responsible for the antelope disease. The results of the HainLifescience CM test and mass spectrometry indicated that the mycobacterium responsible for causing mycobacteriosis was M. avium. New Generation Sequencing helped to identified a subspecies that was M. avium ssp. hominissuis.
Conclusions:
The sitatunga antelope is an animal susceptible to infection by M. avium ssp. hominissuis. Considering the wide range of hosts and the easiness of interspecies transmission of the pathogen, as well as its zoonotic nature, the mycobacteriosis induced by this microorganism should not be underestimated.
Mycobacteriosis are diseases caused by acid-fast mycobacteria other than M. leprae and tuberculous mycobacteria. Animal mycobacteriosis is often caused by M. avium ssp. hominissuis. Many species of animals are susceptible to infection with this bacterium, even those kept in Zoological Gardens. The aim of the study was to determine the species of bacterium responsible for causing the disease in the tested animals.
Material and methods:
Tissue samples of two male sitatunga antelopes (Tragelaphus spekii) were analyzed. Lymph node and lung samples were subjected to anatomical examination and Ziehl-Neelsen staining. Real-time PCR was performed to confirm or rule out tuberculosis mycobacteria infection. In order to isolate the bacterial strain, tissue samples were inoculated on both solid and liquid media. HainLifescience CM tests, mass spectrometry and New Generation Sequencing were used to determine the mycobacterial species.
Results:
Results showed that atypical mycobacteria are responsible for the antelope disease. The results of the HainLifescience CM test and mass spectrometry indicated that the mycobacterium responsible for causing mycobacteriosis was M. avium. New Generation Sequencing helped to identified a subspecies that was M. avium ssp. hominissuis.
Conclusions:
The sitatunga antelope is an animal susceptible to infection by M. avium ssp. hominissuis. Considering the wide range of hosts and the easiness of interspecies transmission of the pathogen, as well as its zoonotic nature, the mycobacteriosis induced by this microorganism should not be underestimated.
REFERENCES (23)
1.
Ryu YJ, Koh WJ, Daley CL. Diagnosis and Treatment of Nontuberculous Mycobacterial Lung Disease: Clinicians’ Perspectives. Tuberc Respir Dis (Seoul). 2016; 79(2): 74–84. doi: 10.4046/trd.2016.79.2.74.
2.
Sharma SK, Upadhyay V. Epidemiology, diagnosis & treatment of non-tuberculous mycobacterial diseases. Indian J Med Res. 2020; 152(3): 185–226. doi: 10.4103/ijmr.IJMR_902_20.
3.
de Juan L, Alvarez JA, Romero B, et al. Comparison of Four Different Culture Media for Isolation and Growth of Type II and Type I/III Mycobacterium avium subsp. paratuberculosis Strains Isolated from Cattle and Goats. Appl Environ Microbiol. 2006; 72(9): 5927–5932. doi: 10.1128/AEM.00451-06.
4.
Zweijpfenning SMH, Ingen JV, Hoefsloot W. Geographic Distribution of Nontuberculous Mycobacteria Isolated from Clinical Specimens: A Systematic Review. Semin Respir Crit Care Med. 2018; 39(3): 336–342. doi: 10.1055/s-0038-1660864. Epub 2018 Aug 2.
5.
Asakura T, Nakagawa T, Suzuki S, et al. Nontuberculous Mycobacteriosis Japan Research Consortium (NTM – JRC). Efficacy and safety of intermittent maintenance therapy after successful treatment of Mycobacterium avium complex lung disease. J Infect Chemother. 2019; 25(3): 218–221. doi: 10.1016/j.jiac.2018.07.021. Epub 2018 Aug 29.
6.
Scherrer S, Landolt P, Carroli N, et al. Molecular Characterization of Mycobacterium avium subsp. hominissuis of Two Groups of Lymph Nodes, Being Intradermal Tuberculin or Interferon-Gamma Test Positive and Negative, Isolated from Swiss Cattle at Slaughter. Front Vet Sci. 2018; 32(5). doi: 10.3389/fvets.2018.00032.
7.
Wilińska E, Szturmowicz M. Lung mycobacteriosis – clinical presentation, diagnostics and treatment. Pneumol Alergol Pol. 2010; 78(2): 138–147.
8.
Griffith DE, Aksamit T, Brown-Elliott BA, et al. An official ATS/IDSA statment: diagnosis, treatment and prevention of nontuberculous mycobacterial diseases. Am J Resp Crit Care Med. 2007; 175(4): 367–416. doi: 10.1164/rccm.200604-571ST.
9.
Clinical info HIV.gov. Mycobacterium avium Complex Disease. https://clinicalinfo.hiv.gov/e... (access: 18.08.2021).
10.
Frayne KMF, Chappell BR, Davies JL, et al. Lesions of Mycobacterium avium spp. hominissuis Infection Resembling M. bovis Lesions in a Wild Mule Deer, Canada. Emerg Infect Dis. 2020; 26(7): 1614–1616. doi: 10.3201/eid2607.200187.
11.
Witkowski L, Orłowska B, Rzewuska M, et al. Evidence of low prevalence of mycobacterial lymphadenitis in wild boars (Sus scrofa) in Poland. Acta Vet Scand. 2017; 59(9). doi: 10.1186/s13028-017-0277-0.
12.
Tran V, Liu J, Behr MA. BCG Vaccines. Microbiol Spect. 2014; 2(1). doi: 10.1128/microbiolspec.MGM2-0028-2013.
13.
Goepfert C, Regenscheit N, Schumacher V, et al. Mycobacterium avium subsp. avium Infection in Four Veal Calves: Differentiation from Intestinal Tuberculosis. BioMed Res International. 2014; 2014. http://dx.doi.org/10.1155/2014....
14.
Krajewska-Wędzina M, Dąbrowska A, Augustynowicz-Kopeć E, et al. Nontuberculous mycobacterial skin disease in cat; diagnosis and treatment – Case report. Ann Agric Environ Med. 2019; 26(3): 511–513. doi: 10.26444/aaem/101579.
15.
Moravkova M, Mrlik V, Parmova, I, et al. High incidence of Mycobacterium avium subspecies hominissuis infection in a zoo population of bongo antelopes (Tragelaphus eurycerus). J Vet Diagn Invest. 2013; 25(4): 531–534. doi: 10.1177/1040638713490689.
16.
Marochi-Telles JP, Muniz R, Sztajnbok J, et al. Disseminated Mycobacterium avium on HIV/AIDS: Historical and Current Literature Review. AIDS Rev. 2020; 22(1): 9–15. doi: 10.24875/AIDSRev.20000104.
17.
Lipiec M. Gruźlica bydlęca, rozpoznawanie, zwalczanie, stan obecny, komentarze. PIWet – PIB w Puławach. 2016; 1: 7–121.
18.
Hernández-Jarguín AM, Martínez-Burnes J, Molina-Salinas GM. Isolation and Histopathological Changes Associatedwith Non-Tuberculous Mycobacteria in Lymph Nodes Condemned at a Bovine Slaughterhouse. Vet Sci. 2020; 7(4): 172. doi: 10.3390/vetsci7040172.
19.
Barry C, Corbett D, Bakker D, et al. The Effect of Mycobacterium avium Complex Infections on Routine Mycobacterium bovis Diagnostic Tests. Vet Med Int. 2011; 2011. doi: 10.4061/2011/145092.
20.
Didkowska A, Żmuda P, Kwiecień E, et al. Microbiological assessment of sheep lymph nodes with lymphadenitis found during post-mortem examination of slaughtered sheep: implications for veterinary-sanitary meat control. Acta Vet Scand. 2020; 62(48). doi: 10.1186/s13028-020-00547-x.
21.
Witkowski L, Rzewuska M, Takai S, et al. Molecular epidemiology of Rhodococcus equi in slaughtered swine, cattle and horses in Poland. BMC Microbiol. 2016; 16(98). doi: 10.1186/s12866-016-0712-9.
22.
Witkowski L, Rzewuska M, Cisek AA, et al. Prevalence and genetic diversity of Rhodococcus equi in wild boars (Sus scrofa), roe deer (Capreolus capreolus) and red deer (Cervus elaphus) in Poland. BMC Microbiol. 2015; 15(110). doi: 10.1186/s12866-015-0445-1.
23.
Żychska M, Witkowski L, Klementowska A, et al. Rhodococcus equi – Occurrence in Goats and Clinical Case Report. Pathogens. 2021; 10(9). doi: 10.3390/pathogens10091141.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.