Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
RESEARCH PAPER
Levels of filamentous fungi and selected mycotoxins in leafy and fruit vegetables and analysis of their potential health risk for consumers
1
Department of Health Biohazards and Parasitology, Institute of Rural Health, Lublin, Poland
Corresponding author
Teresa Kłapeć
Department of Health Biohazards and Parasitology, Institute of Rural Health, Jaczewskiego 2,, 20-090, Lublin, Poland
Department of Health Biohazards and Parasitology, Institute of Rural Health, Jaczewskiego 2,, 20-090, Lublin, Poland
Ann Agric Environ Med. 2021;28(4):585-594
KEYWORDS
TOPICS
ABSTRACT
Introduction and objective:
The aim of the study was to determine the presence, concentration and generic composition of filamentous fungi. Considering the significant role of mycotoxins in the pathogenicity of fungal contaminants of vegetables, the scope of the study was extended by determination of aflatoxins and deoxynivalenol.
Material and methods:
In the years 2019–2020, samples of vegetables (lettuce, spinach, tomato, red pepper) collected on conventional farms located in eastern Poland were subjected to mycological examination. The concentration and species composition of filamentous fungi were determined by the method of plate dilutions on malt agar. The isolated strains were identified with the use of macroscopic and microscopic methods. Samples were also analyzed for the presence of aflatoxin B1 (AFB1), total aflatoxin (AFT) and deoxynivalenol (DON) using the immunoenzymatic ELISA method.
Results:
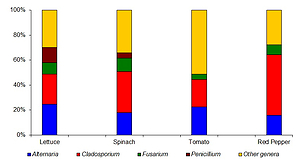
The median concentrations of filamentous fungi ranged from 2.778–3.204 log10 CFU g -1. Overall, 40 fungal species were identified in the examined vegetables, of which 38 are classified as potentially pathogenic for humans. The mean prevalence values for AFB1 and AFT were moderate or high (16.0–60.0% and 57.8–75.6%, respectively) and very low for DON (0–2.2%). The median concentrations of filamentous fungi, AFB1 and AFT were distinctly greater in leafy vegetables than on non-leafy tomato and pepper fruits, and the differences were highly significant (P<0.001).
Conclusions:
The levels of filamentous fungi and mycotoxins in Polish vegetables could be classified as moderate or low. The abundant presence of species with various pathogenic abilities may pose a risk for some categories of people consuming raw vegetables, mostly for immuno-compromised persons or atopics susceptible to food allergy caused by ingested moulds.
The aim of the study was to determine the presence, concentration and generic composition of filamentous fungi. Considering the significant role of mycotoxins in the pathogenicity of fungal contaminants of vegetables, the scope of the study was extended by determination of aflatoxins and deoxynivalenol.
Material and methods:
In the years 2019–2020, samples of vegetables (lettuce, spinach, tomato, red pepper) collected on conventional farms located in eastern Poland were subjected to mycological examination. The concentration and species composition of filamentous fungi were determined by the method of plate dilutions on malt agar. The isolated strains were identified with the use of macroscopic and microscopic methods. Samples were also analyzed for the presence of aflatoxin B1 (AFB1), total aflatoxin (AFT) and deoxynivalenol (DON) using the immunoenzymatic ELISA method.
Results:
The median concentrations of filamentous fungi ranged from 2.778–3.204 log10 CFU g -1. Overall, 40 fungal species were identified in the examined vegetables, of which 38 are classified as potentially pathogenic for humans. The mean prevalence values for AFB1 and AFT were moderate or high (16.0–60.0% and 57.8–75.6%, respectively) and very low for DON (0–2.2%). The median concentrations of filamentous fungi, AFB1 and AFT were distinctly greater in leafy vegetables than on non-leafy tomato and pepper fruits, and the differences were highly significant (P<0.001).
Conclusions:
The levels of filamentous fungi and mycotoxins in Polish vegetables could be classified as moderate or low. The abundant presence of species with various pathogenic abilities may pose a risk for some categories of people consuming raw vegetables, mostly for immuno-compromised persons or atopics susceptible to food allergy caused by ingested moulds.
REFERENCES (98)
1.
Jung Y, Jang H, Matthews KR. Effect of the food production chain from farm practices to vegetable processing on outbreak incidence. Microb Biotechnol. 2014; 7(6): 517–527. https://doi.org/10.1111/1751-7....
2.
Mason-D’Croz D, Bogard JR, Sulser TB, et al. Gaps between fruit and vegetable production, demand, and recommended consumption at global and national levels: an integrated modelling study. Lancet Planet Health. 2019; 3(7): e318–e329. https://doi.org/10.1016/S2542-....
3.
Berger CN, Sodha SV, Shaw RK, et al. Fresh fruit and vegetables as vehicles for the transmission of human pathogens. Environ Microbiol. 2010; 12(9): 2385–2397. https://doi.org/10.1111/j.1462....
4.
Kovács M. Nutritional health aspects of mycotoxins. Orv Hetil. 2004; 145(34): 1739–1746.
5.
Lugauskas A, Repečkienė J, Novošinskas H. Micromycetes, producers of toxins, detected on stored vegetables. Ann Agric Environ Med. 2005; 12(2): 253–260.
6.
Tournas VH. Moulds and yeasts in fresh and minimally processed vegetables, and sprouts. Int J Food Microbiol. 2005; 99(1): 71–77. https://doi.org/10.1016/j.ijfo....
7.
Marinelli L, Maggi O, Aurigemma C, et al. Fresh vegetables and ready-to eat salad: phenotypic characterization of moulds and molecular characterization of yeasts. Ann Ig. 2012; 24(4): 301–309.
8.
Alshannaq A, Yu JH. Occurrence, toxicity, and analysis of major mycotoxins in food. Int J Environ Res Public Health. 2017; 14(6): 632. https://doi.org/10.3390/ijerph....
9.
Ráduly Z, Szabó L, Madar A, et al. Toxicological and medical aspects of Aspergillus-derived mycotoxins entering the feed and food chain. Front Microbiol. 2020; 10: 2908. https://doi.org/10.3389/fmicb.....
10.
Medina A, Rodriguez A. Editorial: Special Issue on environmental changes and mycotoxins. Fungal Biol. 2020; 125(2): 77. https://doi.org/10.1016/j.funb....
11.
Barkai-Golan R, Paster N. Mycotoxins in fruits and vegetables. Academic Press, 2008.
13.
Reddy KRN, Salleh B, Saad B, et al. An overview of mycotoxin contamination in foods and its implications for human health. Toxin Rev. 2010; 29(1): 3–26. https://doi.org/10.3109/155695....
14.
Thunberg RL, Tran TT, Bennett RW, et al. Microbial evaluation of selected fresh produce obtained at retail markets. J Food Prot. 2002; 65(4): 677–682. https://doi.org/10.4315/0362-0....
15.
Abadias M, Usall J, Anguera M, et al. Microbiological quality of fresh, minimally-processed fruit and vegetables, and sprouts from retail establishments. Int J Food Microbiol. 2008; 123(1–2): 121–129. https://doi.org/10.1016/j.ijfo....
16.
Badosa E, Trias R, Parés D, et al. Microbiological quality of fresh fruit and vegetable products in Catalonia (Spain) using normalised plate counting methods and real time polymerase chain reaction (QPCR). J Sci Food Agric. 2008; 88(4): 605–611. https://doi.org/10.1002/jsfa.3....
17.
Oliveira M, Usall J, Viñas I, et al. Microbiological quality of fresh lettuce from organic and conventional production. Food Microbiol. 2010; 27(5): 679–684. https://doi.org/10.1016/j.fm.2....
18.
Maffei DF, De Arruda Silveira NF, da Penha Longo Mortatti Catanozi M. Microbiological quality of organic and conventional vegetables sold in Brazil. Food Control. 2013; 29(1): 226–230. https://doi.org/10.1016/j.food....
19.
Buyukunal SK, Issa G, Aksu F, et al. Microbiological quality of fresh vegetables and fruits collected from supermarkets in Istanbul, Turkey. J Food Nutr Sci. 2015; 3(4): 152–159. https://doi.org/10.11648/j.jfn....
20.
Kuan CH, Rukayadi Y, Ahmad SH, et al. Comparison of the microbiological quality and safety between conventional and organic vegetables sold in Malaysia. Front Microbiol. 2017; 8: 1433. https://doi.org/10.3389/fmicb.....
21.
Szczech M, Kowalska B, Smolińska U, et al. Microbial quality of organic and conventional vegetables from Polish farms. Int J Food Microbiol. 2018; 286: 155–161. https://doi.org/10.1016/j.ijfo....
22.
Acevedo L, Mendoza C, Oyón R. Total and fecal coliforms, some enterobacteria, staphylococcus sp. and moulds in salads for hot dogs sold in Maracay, Venezuela. Arch Latinoam Nutr. 2001; 51(4): 366–370.
23.
Jeddi MZ, Yunesian M, Gorji ME, et al. Microbial evaluation of fresh, minimally-processed vegetables and bagged sprouts from chain supermarkets. J Health Popul Nutr. 2014; 32(3): 391–399.
24.
Ham H, Kim S, Kim MH, et al. Mycobiota of ground red pepper and their aflatoxigenic potential. J Microbiol. 2016; 54(12): 832–837. https://doi.org/10.1007/s12275....
25.
Kłapeć T, Cholewa G, Cholewa A, et al. Fungal diversity of root vegetables and soil rhizosphere collected from organic and conventional farms in Eastern Poland. Ann Agric Environ Med. 2018; 25(2): 374–381. https://doi.org/10.26444/aaem/....
26.
Asai T, Tsuchiya Y, Okano K, et al. Aflatoxin contamination of red chili pepper from Bolivia and Peru, countries with high gallbladder cancer incidence rates. Asian Pac J Cancer Prev. 2012; 13(10): 5167–5170. https://doi.org/10.7314/apjcp.....
27.
Singh P, Cotty PJ. Aflatoxin contamination of dried red chilies: Contrasts between the United States and Nigeria, two markets differing in regulation enforcement. Food Control. 2017; 80: 374–379. https://doi.org/10.1016/j.food....
28.
Casquete R, Rodríguez A, Hernández A, et al. Occurrence of toxigenic fungi and mycotoxins during smoked paprika production. J Food Prot. 2017; 80(12): 2068–2077. https://doi.org/10.4315/0362-0....
29.
Hacıbekiroğlu I, Kolak U. Aflatoxins in various food from Istanbul, Turkey. Food Addit Contam. Part B Surveill. 2013; 6: 260–264. https://doi.org/10.1080/193932....
30.
Hariprasad P, Durivadivel P, Snigdha M. Natural occurrence of aflatoxin in green leafy vegetables. Food Chem. 2013; 138(2–3): 1908–1913. https://doi.org/10.1016/j.food....
31.
Carballo D, Font G, Ferrer E, et al. Evaluation of mycotoxin residues on ready-to-eat food by chromatographic methods coupled to mass spectrometry in tandem. Toxins. 2018; 10(6): 243. https://doi.org/10.3390/toxins....
32.
Samson RA, Houbraken J, Frisvad JC, et al. Food and Indoor Fungi. CBS-KNAW, Fungal Biodiversity Centre, 2010.
34.
Krzyściak P, Skóra M, Macura AB. Atlas grzybów chorobotwórczych człowieka. 1st ed. MedPharm; 2011.
35.
Hilmioglu S, Metin DY, Tasbakan M, et al. Skin infection on both legs caused by Acremonium strictum (case report). Ann Saudi Med. 2015; 35(5): 406–408. https://doi.org/10.5144/0256-4....
36.
Escrivá L, Oueslati S, Font G, et al. Alternaria mycotoxins in food and feed: an overview. J Food Quality. 2017; ID 1569748(20). https://doi.org/10.1155/2017/1....
37.
Zwickel T, Kahl SM, Rychlik M, et al. Chemotaxonomy of mycotoxigenic small-spored Alternaria fungi – Do multitoxin mixtures act as an indicator for species differentiation? Front Microbiol. 2018; 9: 1368. https://doi.org/10.3389/fmicb.....
38.
Skóra J, Otlewska A, Gutarowska B, et al. Production of the allergenic protein Alt a 1 by Alternaria isolates from working environments. Int J Environ Res. Public Health. 2015; 12: 2164–2183. https://doi.org/10.3390/ijerph....
39.
Romano C, Valenti L, Miracco C, et al. Two cases of cutaneous phaeohyphomycosis by Alternaria alternata and Alternaria tenuissima. Mycopathologia. 1997; 137: 65–74. https://doi.org/10.1023/a:1006....
40.
Hu W, Ran Y, Zhuang K, et al. Alternaria arborescens infection in a healthy individual and literature review of cutaneous alternariosis. Mycopathologia. 2015; 179(1–2): 147–152. https://doi.org/10.1007/s11046....
41.
Kieselová K, Gomes T, Santiago F, et al. Emerging cutaneous phaeohyphomycosis caused by Alternaria infectoria. Acta Med Port. 2020; 33(13). https://doi.org/10.20344/amp.1....
42.
Lacey J, Dutkiewicz J. Bioaerosols and occupational lung disease. J Aerosol Sci. 1994; 25(8): 1371–1404. https://doi.org/10.1016/0021-8....
43.
Selman M, Pardo A, King TEJr, et al. Hypersensitivity pneumonitis: insights in diagnosis and pathobiology. Am J Respir Crit Care Med. 2012; 186(4): 314–324. https://doi.org/10.1164/rccm.2....
44.
Hedayati MT, Pasqualotto AC, Warn PA. Aspergillus flavus : human pathogen, allergen and mycotoxin producer. Microbiology. 2007; 153(Pt6): 1677–1692. https://doi.org/10.1099/mic.0.....
45.
Latgé JP, Chamilos G. Aspergillus fumigatus and aspergillosis in 2019. Clin Microbiol Rev. 2019; 33(1): e00140–18. https://doi.org/10.1128/CMR.00....
46.
Frisvad JC, Møller LLH, Larsen TO, et al. Safety of the fungal workhorses of industrial biotechnology: update on the mycotoxin and secondary metabolite potential of Aspergillus niger, Aspergillus oryzae, and Trichoderma reesei. Appl Microbiol Biotechnol. 2018; 102(22): 9481–9515. https://doi.org/10.1007/s00253....
47.
Mishra GS, Mehta N, Pal M. Chronic bilateral otomycosis caused by Aspergillus niger. Mycoses. 2004; 47(1–2): 82–84. https://doi.org/10.1046/j.0933....
48.
Person AK, Chudgar SM, Norton BL, et al. Aspergillus niger: an unusual cause of invasive pulmonary aspergillosis. J Med Microbiol. 2010; 59(Pt7): 834–838. https://doi.org/10.1099/jmm.0.....
49.
Paterson RRM, Lima N. Filamentous fungal human pathogens from food emphasizing Aspergillus, Fusarium and Mucor. Microorganisms. 2017; 5(3): 44. doi: 10.3390/microorganisms5030044.
50.
Varga J, Baranyi N, Chandrasekaran M, et al. Mycotoxin producers in the Aspergillus genus: an update. Acta Biol Szeged. 2015; 59(2): 151–167.
51.
Luo Y, Li J, Zhang X, et al. Characterization of potential pathogenic Cladosporium exposure risks from heating, ventilation and air conditioning (HVAC) in two cities, China. Med Mycol Open Access. 2016; 2: 18. https://doi.org/10.21767/2471-....
52.
Nath R, Barua S, Barman J, et al. Subcutaneous mycosis due to Cladosporium cladosporioides and Bipolaris cynodontis from Assam, North-East India and review of published literature. Mycopathologia. 2015; 180(5–6): 379–387. https://doi.org/10.1007/s11046....
53.
Yew SM, Chan CL, Ngeow YF, et al. Insight into different environmental niches adaptation and allergenicity from the Cladosporium sphaerospermum genome, a common human allergy-elicitingDothideomycetes. Sci Rep. 2016; 6: 27008. https://doi.org/10.1038/srep27....
54.
Batra N, Kaur H, Mohindra S, et al. Cladosporium sphaerospermum causing brain abscess, a saprophyte turning pathogen: case and review of published reports. J Mycol Med. 2019; 29(2): 180–184. https://doi.org/10.1016/j.mycm....
55.
Nucci M, Anaissie E, et al. Fusarium infections in immunocompromised patients. Clin Microbiol Rev. 2007; 20(4): 695–704. https://doi.org/10.1128/CMR.00....
56.
Sun S, Lui Q, Han L, et al. Identification and characterization of Fusarium proliferatum, a new species of fungi that cause fungal keratitis. Sci Rep. 2018; 8(1): 4859. https://doi.org/10.1038/s41598....
57.
Stenglein SA. Fusarium poae: A pathogen that needs more attention. J Plant Pathol. 2009; 91(1): 25–36. http://dx.doi.org/10.4454/jpp.....
58.
Zentai A, Szeitzné-Szabó M, Mihucz G, et al. Occurrence and risk assessment of fumonisin B1 and B2 mycotoxins in maize-based food products in Hungary. Toxins. 2019; 11(12): 709. http://doi.org/10.3390/toxins1....
59.
Barral B, Chillet M, Doizy A, et al. Diversity and toxigenicity of fungi that cause pineapple fruitlet core rot. Toxins. 2020; 12(5): 339. https://doi.org/10.3390/toxins....
60.
El-Banna AA, Scott PM, Lau PY, et al. Formation of trichothecenes by Fusarium solani var. coeruleum and Fusarium sambucinum in potatoes. Appl Environ Microbiol. 1984; 47(5): 1169–1171. https://doi.org/10.1128/aem.47....
61.
Xue HL, Bi Y, Tang YM, et al. Effect of cultivars, Fusarium strains and storage temperature on trichothecenes production in inoculated potato tubers. Food Chem. 2014; 151: 236–242. https://doi.org/10.1016/j.food....
62.
Henrich TJ, Marty FM, Milner DA Jr, et al. Disseminated Geotrichum candidum infection in a patient with relapsed acute myelogenous leukemia following allogeneic stem cell transplantation and review of the literature. Transpl Infect Dis. 2009; 11(5): 458–462. https://doi.org/10.1111/j.1399....
63.
Ostrosky-Zeichner L, Sobel JD. Fungal Infections. Infect Dis Clin North Am. 2016; 30(1): XIII-XIV. https://doi.org/10.1016/S0891-....
64.
Ziaee A, Zia M, Bayat M, et al. Molecular identification of Mucor and Lichtheimia species in pure cultures of Zygomycetes. Jundishapur J Microbiol. 2016; 9(4): e35237. https://doi.org/10.5812/jjm.35....
65.
Levetin E, Horner WE, Scott JA, et al. Taxonomy of allergenic fungi. J Allergy Clin Immunol Pract. 2016; 4(3): 375–385.e1. https://doi.org/10.1016/j.jaip....
66.
Biango-Daniels MN, Wang TW, Hodge KT. Draft genome sequence of the patulin-producing fungus Paecilomyces niveus strain CO7. Genome Announc. 2018; 6(25): e00556–18. https://doi.org/10.1128/genome....
67.
Hara J, Fujimura M, Tachibana H, Myou, et al. A case of acute hypersensitivity pneumonitis associated with an oil fan heater. Am J Med Sci. 2006; 331(1): 35–36. https://doi.org/10.1097/000004....
68.
Su M, Zhao C, Li D, et al. Viriditoxin stabilizes microtubule polymers in SK-OV-3 Cells and exhibits antimitotic and antimetastatic potential. Mar Drugs. 2020; 18(9): 445. https://doi.org/10.3390/md1809....
69.
Moreira DC, Oliveira MME, Borba CM. Human pathogenic Paecilomyces from food. Microorganisms. 2018; 6(3): 64. https://doi.org/10.3390/microo....
70.
Sprute R, Salmanton-García J, Sal E, et al. Characterization and outcome of invasive infections due to Paecilomyces variotii: analysis of patients from the FungiScope® registry and literature reports. J Antimicrob Chemother. 2021; 76(3): 765–774. https://doi.org/10.1093/jac/dk....
71.
Okano T, Kobayashi N, Izawa K, et al. Whole genome analysis revealed the genes responsible for citreoviridin biosynthesis in Penicillium citreonigrum. Toxins. 2020; 12(2): 125. https://doi.org/10.3390/toxins....
72.
Frisvad JC, Samson RA, Rassing BR, et al. Penicillium discolor, a new species from cheese, nuts and vegetables. Antonie Van Leeuwenhoek. 1997; 72(2): 119–126. https://doi.org/10.1023/a:1000....
73.
Vega FE, Posada F, Peterson SW, et al. Penicillium species endophytic in coffee plants and ochratoxin A production. Mycologia. 2006; 98(1): 31–42. https://doi.org/10.3852/mycolo....
74.
Ueno Y, Sato N, Ito T, et al. Chronic toxicity and hepatocarcinogenicity of (+) rugulosin, an anthraquinoid mycotoxin from Penicillium species: preliminary surveys in mice. J Toxicol Sci. 1980; 5(4): 295–302. https://doi.org/10.2131/jts.5.....
75.
Skóra M, Bielecki J, Bulanda M, et al. Fungi of the genus Scopulariopsis – ill-defined human pathogens. Post Mikrobiol. 2015; 54(1): 44–52.
76.
McCormick SP, Stanley AM, Stover NA. Trichothecenes: from simple to complex mycotoxins. Toxins. 2011; 3(7): 802–814. https://doi.org/10.3390/toxins....
77.
Richter S, Cormican MG, Pfaller MA, et al. Fatal disseminated Trichoderma longibrachiatum infection in an adult bone marrow transplant patient: species identification and review of the literature. J Clin Microbiol. 1999; 37(40): 1154–1160. https://doi.org/10.1128/JCM.37....
78.
Kantarcioglu AS, Celkan T, Yücel A, et al. Fatal Trichoderma harzianum infection in a leukemic pediatric patient. Med Mycol. 2009; 47(2): 207–215. https://doi.org/10.1080/136937....
79.
Weinhold B. “Trilongins” offer insight into mold toxicity. Environ Health Perspect. 2013; 121(2): a44. https://doi.org/10.1289/ehp.12....
80.
Recio R, Melendez-Carmona MÁ, Martín-Higuera MC, et al. Trichoderma longibrachiatum: an unusual pathogen of fungal pericarditis. Clin Microbiol Infect. 2019; 25(5): 586–587. https://doi.org/10.1016/j.cmi.....
81.
Hatvani L, Homa M, Chenthamara K, et al. Agricultural systems as potential sources of emerging human mycoses caused by Trichoderma: a successful, common phylotype of Trichoderma longibrachiatum in the frontline. FEMS Microbiol Lett. 2019; 366(21): fnz246. https://doi.org/10.1093/femsle....
82.
Hou CT, Ciegler A, Hesseltine CW. New mycotoxin, trichotoxin A, from Trichoderma viride isolated from southern leaf blight-infected corn. Appl Microbiol. 1972; 23(1): 183–185. https://doi.org/10.1128/am.23.....
83.
Kespohl S, Raulf M. Mould allergens: where do we stand with molecular allergy diagnostics? Part 13 of the series Molecular Allergology. Allergol J Int. 2014; 23(4): 120–125. https://doi.org/10.1007/s40629....
84.
Bennett A, Ponder MM, Garcia-Diaz J. Phoma infections: classification, potential food sources, and their clinical impact. Microorganisms. 2018; 6(3): 58. https://doi.org/10.3390/microo....
85.
Costa J, Rodríguez R, Garcia-Cela E, et al. Overview of fungi and mycotoxin contamination in Capsicum pepper and in its derivatives. Toxins. 2019; 11(1): 27. https://doi.org/10.3390/toxins....
86.
Luciano-Rosario D, Keller NP, Jurick WM. Penicillium expansum: biology, omics, and management tools for a global postharvest pathogen causing blue mould of pome fruit. Mol Plant Pathol. 2020; 21: 1391–1404. https://doi.org/10.1111/mpp.12....
87.
Benavidez Rozo ME, Patriarca A, Cabrera G, et al. Determination of the profiles of secondary metabolites characteristic of Alternaria strains isolated from tomato. Rev Iberoam Micol. 2014; 31(2): 119–124. https://doi.org/10.1016/j.riam....
88.
Van de Perre E, Deschuyffeleer N, Jacxsens L, et al. Screening of moulds and mycotoxins in tomatoes, bell peppers, onions, soft red fruits and derived tomato products. Food Control. 2014; 37: 165–170. https://doi.org/10.1016/j.food....
89.
Da Cruz Cabral L, Terminiello L, Fernández Pinto V, et al. Natural occurrence of mycotoxins and toxigenic capacity of Alternaria strains from mouldy peppers. Int J Food Microbiol. 2016; 236: 155–160. https://doi.org/10.1016/j.ijfo....
90.
Solfrizzo M, De Girolamo A, Vitti C, et al. Liquid chromatographic determination of Alternaria toxins in carrots. J AOAC Int. 2004; 87(1): 101–106.
91.
Alwatban MA, Hadi S, Moslem MA. Mycotoxin production in Cladosporium species influenced by temperature regimes. J Pure Appl Microbiol. 2014; 8(5): 4061–4069.
92.
Corrier DE. Mycotoxicosis: mechanisms of immunosuppression. Vet Immunol Immunopathol. 1991; 30(1): 73–87. https://doi.org/10.1016/0165-2....
93.
EFSA Panel on Contaminants in the Food Chain (CONTAM), Schrenk D, Bignami M, et al. Risk assessment of aflatoxins in food. EFSA J. 2020; 18(3): e06040. https://doi.org/10.2903/j.efsa....
94.
Alassane-Kpembi I, Kolf-Clauw M, Gauthier T, et al. New insights into mycotoxin mixtures: the toxicity of low doses of Type B trichothecenes on intestinal epithelial cells is synergistic. Toxicol Appl Pharmacol. 2013; 272(1): 191–198. https://doi.org/10.1016/j.taap....
95.
Popescu FD. Cross-reactivity between aeroallergens and food allergens. World J Methodol. 2015; 5(2): 31–50. https://doi.org/10.5662/wjm.v5....
96.
Luccioli S, Malka-Rais J, Nsnuli TM, et al. Clinical reactivity to ingestion challenge with mixed mold extract may be enhanced in subjects sensitized to molds. Allergy Asthma Proc. 2009; 30(4): 433–442. https://doi.org/10.2500/aap.20....
97.
Schütze N, Lehmann I, Bönisch U, et al. Exposure to mycotoxins increases the allergic response in a murine asthma model. Am J Respir Crit Care Med. 2010; 181(11): 1188–1199. https://doi.org/10.1164/rccm.2....
98.
European Commission. Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Official Journal of the European Union, L 364/5, 2006.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.