Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
RESEARCH PAPER
Dihydroergotamine affects spatial behavior and neurotransmission in the central nervous system of Wistar rats
1
Centre for Preclinical Research and Technology CePT, Medical University, Warsaw, Poland
Corresponding author
Justyna Pyrzanowska
Medical University of Warsaw Centre for Preclinical Research and Technology CePT, Banacha 1b, 02-097, Warszawa, Poland
Medical University of Warsaw Centre for Preclinical Research and Technology CePT, Banacha 1b, 02-097, Warszawa, Poland
Ann Agric Environ Med. 2021;28(3):437-445
KEYWORDS
TOPICS
ABSTRACT
Introduction:
Dihydroergotamine (DHE) is a derivative of an ergot alkaloid used as an antimigraine medication. Nowadays, ergot alkaloids may still endanger the safety of humans and animals as food or medicine pollutants, but the outcomes of long-term DHE administration on the behaviour and neurotransmission remain undescribed.
Material and methods:
Adult male Wistar Albino Glaxo rats pre-treated orally with DHE for six weeks were investigated to assess the relationship between concentration of neurotransmitters and behavioural response. The behavioural effects of the drug administered at doses of either 30 µg/kg b.w. (group DHE30, n = 11) or 100 µg/kg b.w. per day (group DHE100, n = 10) were evaluated in the Morris Water Maze. It is known that monoaminergic neurotransmitters (serotonin, noradrenaline and dopamine) in some brain structures (prefrontal cortex, hippocampus, striatum, cerebellum, spinal cord) play a role in the control of cognitive and motor functions. The concentration of neurotransmitters was determined by High Performance Liquid Chromatography (HPLC).
Results:
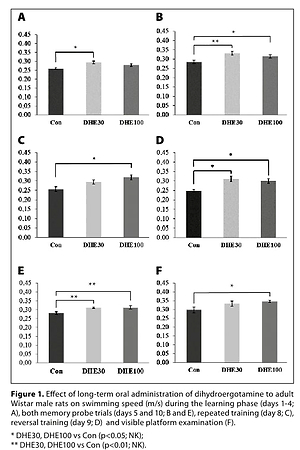
Administration of DHE influenced neither the learning processes nor memory in rats. Nevertheless, an increased motor activity of the DHE-administered animals was observed in both the cued and non-cued behavioural tasks. In HPLC examination, changes in the concentration of monoaminergic neurotransmitters and their metabolites were noted in all tested structures, except for the hippocampus.
Conclusions:
DHE is able to modulate noradrenergic, serotonergic and dopaminergic neurotransmission that may support the increase in locomotion.
Dihydroergotamine (DHE) is a derivative of an ergot alkaloid used as an antimigraine medication. Nowadays, ergot alkaloids may still endanger the safety of humans and animals as food or medicine pollutants, but the outcomes of long-term DHE administration on the behaviour and neurotransmission remain undescribed.
Material and methods:
Adult male Wistar Albino Glaxo rats pre-treated orally with DHE for six weeks were investigated to assess the relationship between concentration of neurotransmitters and behavioural response. The behavioural effects of the drug administered at doses of either 30 µg/kg b.w. (group DHE30, n = 11) or 100 µg/kg b.w. per day (group DHE100, n = 10) were evaluated in the Morris Water Maze. It is known that monoaminergic neurotransmitters (serotonin, noradrenaline and dopamine) in some brain structures (prefrontal cortex, hippocampus, striatum, cerebellum, spinal cord) play a role in the control of cognitive and motor functions. The concentration of neurotransmitters was determined by High Performance Liquid Chromatography (HPLC).
Results:
Administration of DHE influenced neither the learning processes nor memory in rats. Nevertheless, an increased motor activity of the DHE-administered animals was observed in both the cued and non-cued behavioural tasks. In HPLC examination, changes in the concentration of monoaminergic neurotransmitters and their metabolites were noted in all tested structures, except for the hippocampus.
Conclusions:
DHE is able to modulate noradrenergic, serotonergic and dopaminergic neurotransmission that may support the increase in locomotion.
REFERENCES (51)
1.
Baltic Sea Pharmaceuticals in the aquatic environment of the Baltic Sea region. A status report. Baltic Sea Environment Proceedings No. 149. The United Nations Educational, Scientific and Cultural Organization 7, place de Fontenoy, 75352 Paris 07 SP, France and HELCOM. UNESCO 2017. http://www.helcom.fi/Lists/Pub....
2.
Hanoun N, Saurini F, Lanfumey L, Hamon M, Bourgoin S. Dihydroergotamine and its metabolite, 8’-hydroxy-dihydroergotamine, as 5-HT1A receptor agonists in the rat brain. Br J Pharmacol. 2003; 139(2): 424–434. https://doi.org/10.1038/sj.bjp....
3.
Bigal ME, Tepper SJ. Ergotamine and dihydroergotamine: a review. Curr. Pain Headache Rep. 2003; 7(1): 55–62. https://doi.org/10.1007/s11916....
4.
González-Hernández A, Lozano-Cuenca J, Marichal-Cancino BA, Maassen Van Den Brink A, Villalón CM. Dihydroergotamine inhibits the vasodepressor sensory CGRPergic outflow by prejunctional activation of α2-adrenoceptors and 5-HT1 receptors. J Headache Pain. 2018; 19(1): 40. https://doi.org/10.1186/s10194....
5.
Deliganis AV, Peroutka SJ. 5-Hydroxtryptamine1D receptor agonism predicts antimigraine efficacy. Headache. 1991; 31(4): 228–231. https://doi.org/10.1111/j.1526....
6.
Harriott AM, Strother LC, Vila-Pueyo M, Holland PR. Animal models of migraine and experimental techniques used to examine trigeminal sensory processing. J Headache Pain. 2019; 20(1): 91
7.
Masterson CG, Durham PL. DHE repression of ATP-mediated sensitization of trigeminal ganglion neurons. Headache. 2010; 50(9): 1424–1439. https://doi.org/10.1111/j.1526....
8.
American Headache Society. The American Headache Society Position Statement On Integrating New Migraine Treatments Into Clinical Practice. Headache. 2019; 59(1): 1–18. https://doi.org/10.1111/head.1....
9.
Piechal A, Blecharz-Klin K, Mirowska-Guzel D. Dihydroergotamine (DHE) – Is there a place for its use? J Pre-Clin Clin Res. 2018; 12(4): 149–157. https://doi.org/10.26444/jpccr....
10.
Silberstein SD, Shrewbury SB, Hoekman J. Dihydroergotamine (DHE) – then and now: a narrative review. Headache, 2019. https://doi.org/10.1111/head.1....
11.
Saper JR, Silberstein S, Dodick D, Rapoport A. DHE in the pharmacotherapy of migraine: potential for a larger role. Headache. 2006; 46 (Suppl 4): S212–S220. https://doi.org/10.1111/j.1526....
12.
European Medicine Agency. CHMP referral assessment report: Ergot derivatives containing medicinal products. 2013, EMA/750626/2013. https://www.ema.europa.eu/en/d.... Accessed April 12, 2019.
13.
Fioravanti M, Flicker L. Efficacy of nicergoline in dementia and other age associated forms of cognitive impairment. Cochrane Database Syst Rev. 2001; 4: CD003159. https://doi.org/10.1002/146518....
14.
Saletu B, Garg A, Shoeb A. Safety of nicergoline as an agent for management of cognitive function disorders. Biomed Res Int. 2014; 2014: 610103. http://dx.doi.org/10.1155/2014....
15.
Fioravanti M, Nakashima T, Xu J, Garg A. A systematic review and meta-analysis assessing adverse event profile and tolerability of nicergoline. BMJ Open. 2014; 4(7): e005090 (2014). http://dx.doi.org/10.1136/bmjo....
16.
Feldman RS, Meyer JS, Quenzer LF. Neurotransmitter systems. In: Principles of Neuropsychopharmacology, Sinauer Associates, Inc., Publishers, Sunderland, Massachusetts; 1997. pp. 277–390.
17.
Saper JR, Silberstein SD. Pharmacology of dihydroergotamine and evidence for efficacy and safety in migraine. Headache. 2006; 46(Suppl.4): S171–S181. DOI: 10.1111/j.1526–4610.2006.00601.x.
18.
Ala-Hurula V, Myllyla VV, Arvela P, Kärki NT, Hokkanen E. Systemic availability of ergotamine tartrate after three successive doses and during continuous medication. Eur. J Clin Pharmacol. 1979; 16(5): 355–60. https://doi.org/10.1007/BF0060....
19.
Goadsby PJ, Gundlach AL. Localization of 3H-dihydroergotamine-binding sites in the cat central nervous system: relevance to migraine. Ann Neurol. 1991; 29(1): 91–4. https://doi.org/10.1002/ana.41....
20.
Pradalier A, Lantéri-Minet M, Géraud G, Allain H, Lucas C, Delgado A. The PROMISE study: PROphylaxis of MIgraine with SEglor (dihydro -ergotamine mesilate) in French primary care. CNS Drugs. 2004; 18(15): 1149–63. https://doi.org/10.2165/000232....
21.
Schürks M. Dihydroergotamine: role in the treatment of migraine. Expert Opin Drug Metab Toxicol. 2009; 5(9): 1141–8. https://doi.org/10.1517/174252....
22.
Kayser V, Aubel B, Hamon M, Bourgoin S. The antimigraine 5-HT1B/1D receptor agonists, sumatriptan,zolmitriptan and dihydroergotamine, attenuate pain-related behaviour in a rat model of trigeminal neuropathic pain. Br J Pharmacol. 2002; 137, 1287–97. https://doi.org/10.1038/sj.bjp....
23.
Blecharz-Klin K, Piechal A, Jawna-Zboińska K, Pyrzanowska J, Wawer A, Joniec-Maciejak I, Widy-Tyszkiewicz E. Paracetamol – Effect of early exposure on neurotransmission, spatial memory and motor performance in rats. Behav Brain Res. 2017 Apr 14;323:162–171. https://doi.org/10.1016/j.bbr.....
24.
Widy-Tyszkiewicz E, Scheel-Krüger J, Christensen AV. Enhanced disruptive spatial learning effect after sufentanil in renal hypertensive rats versus normotensive rats. Physiol Behav. 1993 Mar;53(3):467–75. https://doi.org/10.1016/0031–9....
25.
Pyrzanowska J, Piechal A, Blecharz-Klin K, Joniec-Maciejak I, Graikou K, Chinou I, Widy-Tyszkiewicz E. Long-term administration of Greek Royal Jelly improves spatial memory and influences the concentration of brain neurotransmitters in naturally aged Wistar male rats. J Ethnopharmacol. 2014; 155(1): 343–351. https://doi.org/10.1016/j.jep.....
26.
Reed MN, Liu P, Kotilinek LA, Ashe KH. Effect size of reference memory deficits in the Morris water maze in Tg2576 mice. Behav Brain Res. 2010; 212(1): 115–20. https://doi.org/10.1016/j.bbr.....
27.
Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protoc. 2006; 1(2): 848–58. https://doi.org/10.1038/nprot.....
28.
Vorhees CV, Williams MT. Assessing spatial learning and memory in rodents. ILAR Journal. 2014; 55(2): 310–332. https://doi.org/10.1093/ilar/i....
29.
Kandel ER, Schwartz JH, Jessell TM, Siegelbaum SA, Hudspeth AJ. Principles of Neural Science. 5th ed. New York, NY: McGraw-Hill Medical; 2013.
30.
Jordan L, Sławińska U. Chapter 17 – The Brain and Spinal Cord Networks Controlling Locomotion. In: Neuronal Networks in Brain Function, CNS Disorders, and Therapeutics, eds. Faingold CL, Blumenfeld H. Academic Press; 2014. pp. 215–233.
31.
Gatto G, Goulding M. Locomotion Control: Brainstem Circuits Satisfy the Need for Speed. Curr Biol. 2018; 28(6): R256-R259. https://doi.org/10.1016/j.cub.....
32.
Hall DA, Powers JP, Gulley JM. Blockade of D1 dopamine receptors in the medial prefrontal cortex attenuates amphetamine- and methamphetamine-induced locomotor activity in the rat. Brain Res. 2009; 1300: 51–57. https://doi.org/10.1016/j.brai....
33.
Ago Y, Tanaka T, Kita Y, Tokumoto H, Takuma K, Matsuda T. Lithium attenuates methamphetamine-induced hyperlocomotion and behavioral sensitization via modulation of prefrontal monoamine release. Neuropharmacol. 2012; 62(4): 1634–1639. https://doi.org/10.1016/j.neur....
34.
Ago Y, Nakamura S, Kajita N, Uda M, Hashimoto H, Baba A, Matsuda T. Ritanserin reverses repeated methamphetamine-induced behavioral and neurochemical sensitization in mice. Synapse. 2007; 61(9): 757–63. https://doi.org/10.1002/syn.20....
35.
Drouin C, Blanc G, Villégier AS, Glowinski J, Tassin J. Critical role of alpha1-adrenergic receptors in acute and sensitized locomotor effects of D-amphetamine, cocaine, and GBR 12783: influence of preexposure conditions and pharmacological characteristics. Synapse. 2002; 43(1): 51–61. https://doi.org/10.1002/syn.10....
36.
Alcaro A, Huber R, Panksepp J. Behavioral functions of the mesolimbic dopaminergic system: an affective neuroethological perspective. Brain Res Rev. 2007; 56(2): 283–321. https://doi.org/10.1016/j.brai....
37.
Viggiano D. The hyperactive syndrome: Metanalysis of genetic alterations, pharmacological treatments and brain lesions which increase locomotor activity. Behav Brain Res. 2008; 194(1): 1–14. https://doi.org/10.1016/j.bbr.....
38.
Ikeda H, Kamei J, Koshikawa N, Cools AR. Nucleus accumbens and dopamine-mediated turning behavior of the rat: role of accumbalnon-dopaminergic receptors. J Pharmacol Sci. 2012; 120(3): 152–164. https://doi.org/10.1254/jphs.1....
39.
Arias-Carrión O, Stamelou M, Murillo-Rodríguez E, Menéndez-González M, Pöppel E. Dopaminergic reward system: a short integrative review. Int Arch Med. 2010; 3: 24. https://doi.org/10.1186/1755–7....
40.
Wang F, Wan P, Wang W, Xiao B, Jin H, Jin Q. Dopamine in the hippocampal dentate gyrus modulates spatial learning via D1-like receptors. Brain Res Bull. 2019; 144: 101–107. https://doi.org/10.1016/j.brai....
41.
McNamara CG, Dupret D. Two sources of dopamine for the hippocampus. Trends Neurosci. 2017; 40(7): 383–384. https://doi.org/10.1016/j.tins....
42.
Moreno-Castilla P, Pérez-Ortega R, Violante-Soria V, Balderas I, Bermúdez-Rattoni F. Hippocampal release of dopamine and norepinephrine encodes novel contextual information. Hippocampus. 2017; 27(5): 547–557. https://doi.org/10.1002/hipo.2....
43.
Dale E, Pehrson AL, Jeyarajah T, Li Y, Leiser SC, Smagin G, Olsen CK, Sanchez C. Effects of serotonin in the hippocampus: how SSRIs and multimodal antidepressants might regulate pyramidal cell function. CNS Spectr. 2015; 21(2): 143–61. https://doi.org/10.1017/S10928....
44.
Gatto G. and M. Goulding, 2018. Locomotion Control: Brainstem Circuits Satisfy the Need for Speed. Curr Biol. 2018; 28(6): R256-R259. https://doi.org/10.1016/j.cub.....
45.
Jordan L, Sławińska U. Chapter 17 – The Brain and Spinal Cord Networks Controlling Locomotion. In: Neuronal Networks in Brain Function, CNS Disorders, and Therapeutics, eds. Faingold CL, Blumenfeld H. Academic Press; 2014. pp: 215–233.
46.
Sharples SA, Koblinger K, Humphreys JM, Whelan PJ. Dopamine: a parallel pathway for the modulation of spinal locomotor networks. Front. Neural Circuits. 2014; 8: 55. https://doi.org/10.3389/fncir.....
47.
Swann HE, Kempe RB, Van Orden AM, Brumley MR. Serotonergic activation of locomotor behavior and posture in one-day old rats. Behav Brain Res. 2016; 302: 104–14. https://doi.org/10.1016/j.bbr.....
48.
Sławińska U, Majczyński H, Dai Y, Jordan LM. The upright posture improves plantar stepping and alters responses to serotonergic drugs in spinal rats. J Physiol. (Lond.) 2012; 590(7): 1721–1736. https://doi.org/10.1113/jphysi....
49.
Sławińska U, Miazga K, Jordan LM. The role of serotonin in the control of locomotor movements and strategies for restoring locomotion after spinal cord injury. Acta Neurobiol Exp. (Wars) 2014; 74(2): 172–87. PMID: 24993627.
50.
Bortolato M, Chen K, Shih JC. Monoamine oxidase inactivation: from pathophysiology to therapeutics. Adv Drug Deliv Rev. 2008; 60(13–14): 1527–33. https://doi.org/10.1016/j.addr....
51.
Villégier AS, Salomon L, Granon S, Changeux JP, Belluzzi JD, Leslie FM, Tassin JP. Monoamine oxidase inhibitors allow locomotor and rewarding responses to nicotine. Neuropsychopharmacology. 2006; 31(8): 1704–13. https://doi.org/10.1038/sj.npp....
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.