Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
RESEARCH PAPER
Effects of Autoimmune Protocol (AIP) diet on changes in thyroid parameters in Hashimoto’s disease
1
University of Life Sciences, Warsaw, Poland
Corresponding author
Ann Agric Environ Med. 2023;30(3):513-521
KEYWORDS
TOPICS
ABSTRACT
Introduction and objective:
In the scientific world, the nutritional needs of persons with Hashimoto’s autoimmune thyroid disease are discussed, and there is a lot of interest in the autoimmune protocol (AIP). The aim of the study was to check the effects of AIP on thyroid parameters in euthyroid patients with Hashimoto’s disease.
Material and methods:
Among 28 people with Hashimoto’s (including 1 male) the consumption of nutrients, anthropometrics, symptoms of the disease, values of thyroid parameters: FT3, FT4, TSH, thyroid ultrasound and autoimmune aTPO, aTG were analyzed before and after 12 weeks of using the AIP diet. The impact of changes in the consumption of selected nutrients on changes in thyroid biochemical parameters were analyzed using multiple regression models, where the dependent variables of the created models were changes in thyroid biochemical parameters.
Results:
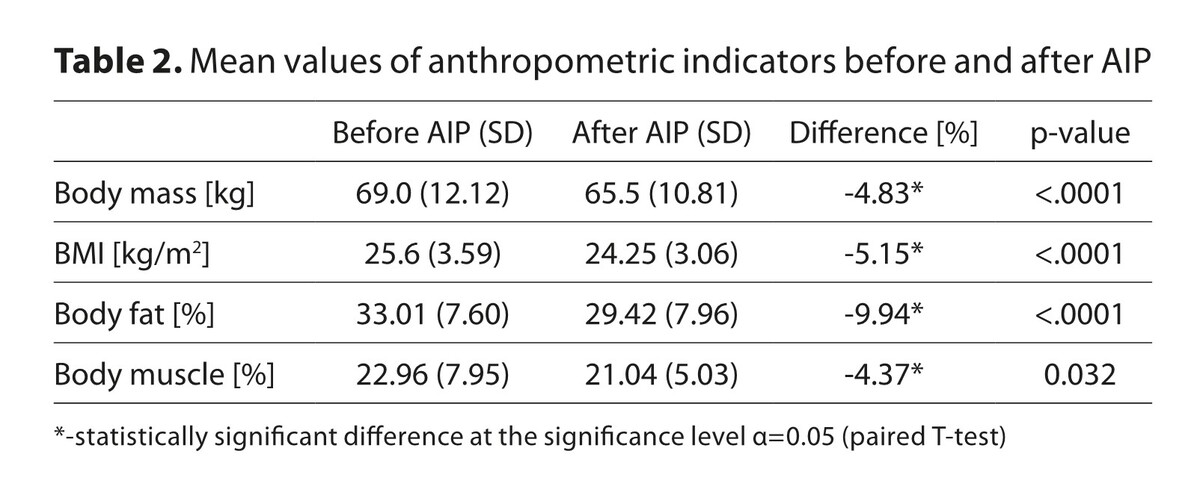
After applying the AIP diet, the number of people reporting symptoms of malaise decreased, the levels of FT3 and FT4 as well as TSH decreased, remaining within the reference concentration range. aTG decreased slightly, but aTPO increased significantly. Ultrasound examination also showed a decrease in the volume of the thyroid gland. The body weight of the subjects decreased, which indicates a caloric deficit.
Conclusions:
Given the numerous advantages of the AIP diet, extending the observation time of the diet, along with its personalization in terms of food selection, energy and nutritional value, could show changes in both well-being and biochemical test results to a greater extent. The use of a personalized AIP protocol can improve the quality of life, a positive change in mental state, reduction of stress, and above all, the improvement of adverse ailments associated with Hashimoto’s disease.
In the scientific world, the nutritional needs of persons with Hashimoto’s autoimmune thyroid disease are discussed, and there is a lot of interest in the autoimmune protocol (AIP). The aim of the study was to check the effects of AIP on thyroid parameters in euthyroid patients with Hashimoto’s disease.
Material and methods:
Among 28 people with Hashimoto’s (including 1 male) the consumption of nutrients, anthropometrics, symptoms of the disease, values of thyroid parameters: FT3, FT4, TSH, thyroid ultrasound and autoimmune aTPO, aTG were analyzed before and after 12 weeks of using the AIP diet. The impact of changes in the consumption of selected nutrients on changes in thyroid biochemical parameters were analyzed using multiple regression models, where the dependent variables of the created models were changes in thyroid biochemical parameters.
Results:
After applying the AIP diet, the number of people reporting symptoms of malaise decreased, the levels of FT3 and FT4 as well as TSH decreased, remaining within the reference concentration range. aTG decreased slightly, but aTPO increased significantly. Ultrasound examination also showed a decrease in the volume of the thyroid gland. The body weight of the subjects decreased, which indicates a caloric deficit.
Conclusions:
Given the numerous advantages of the AIP diet, extending the observation time of the diet, along with its personalization in terms of food selection, energy and nutritional value, could show changes in both well-being and biochemical test results to a greater extent. The use of a personalized AIP protocol can improve the quality of life, a positive change in mental state, reduction of stress, and above all, the improvement of adverse ailments associated with Hashimoto’s disease.
ACKNOWLEDGEMENTS
The study was financially supported by from the resources of
the Polish Ministry of Education and Sciences within funds
of the Institute of Human Nutrition Sciences, University of
Life Sciences (WULS) in Warsaw, for scientific research.
REFERENCES (27)
1.
Ihnatowicz P, Wątor P, Drywień EM. Supplementation in Autoimmune Thyroid Hashimoto’s Disease. Vitamin D and Selenium. J Food Nutr Res. 2019;7(8):584–591. doi:10.12691/jfnr-7-8-6. http://www.sciepub.com/JFNR/ab....
2.
Ihnatowicz P, Drywień M, Wątor P, et al. The importance of nutritional factors and dietary management of Hashimoto’s thyroiditis. Ann Agric Environ Med. 2020;27(2):184–193. doi: 10.26444/aaem/112331.
3.
Wojtas N, Wadolowska L, Bandurska-Stankiewicz E. Evaluation of Qualitative Dietary Protocol (Diet4Hashi) Application in Dietary Counseling in Hashimoto Thyroiditis: Study Protocol of a Randomized Controlled Trial. Int J Environ Res Public Health. 2019;16(23):4841. doi: 10.3390/ijerph16234841.
4.
Trofimiuk-Muldner M, Czubek E, Sztorc J, et al. MON-013 Nutritional Approach To Autoimmune Thyroiditis (AIT) – The Patients’ And Medical Professionals’ View. J Endocr Soc. 2019;3(Suppl 1): MON-013. doi: 10.1210/js.2019-MON-013.
5.
Ihnatowicz P, Wątor P, Drywień ME. The importance of gluten exclusion in the management of Hashimoto’s thyroiditis. Ann Agric Environ Med. 2021;28(4):558–568. doi: 10.26444/aaem/136523.
6.
Szostak-Węgierek D, Bednarczuk T, Respondek W, et al. The validity of using a gluten-free diet in Hashimoto’s disease: the position of the Expert Group of the Medical Dietetics Section of the Polish Society of Parenteral Nutrition and Enteral Metabolism (POLSPEN). Postępy Żywienia Klinicznego. 2018;47:33–47. [in Polish].
7.
Ruggeri RM, Giovinazzo S, Barbalace MC, et al. Influence of Dietary Habits on Oxidative Stress Markers in Hashimoto’s Thyroiditis. Thyroid. 2021;31(1):96–105. doi: 10.1089/thy.2020.0299. Erratum in: Thyroid. 2021;31(4):709.
8.
Frączek B, Pięta A, Burda A, et al. Paleolithic Diet—Effect on the Health Status and Performance of Athletes? Nutrients. 2021;13(3):1019. https://doi.org/10.3390/nu1303....
9.
Martensson A, Stomby A, Tellström A, et al. Using a Paleo Ratio to Assess Adherence to Paleolithic Dietary Recommendations in a Randomized Controlled Trial of Individuals with Type 2 Diabetes. Nutrients. 2021;13(3):969. https://doi.org/10.3390/nu1303....
10.
Liang S, Mijatovic J, Li A, Koemel N, et al. Dietary Patterns and Non-Communicable Disease Biomarkers: A Network Meta-Analysis and Nutritional Geometry Approach. Nutrients. 2023;15(1):76. https://doi.org/10.3390/nu1501....
11.
Ihnatowicz P, Wątor P, Gębski J, et al. Are Nutritional Patterns among Polish Hashimoto Thyroiditis Patients Differentiated Internally and Related to Ailments and Other Diseases? Nutrients. 2021;13(11):3675. doi:10.3390/nu13113675.
12.
Abbott RD, Sadowski A, Alt AG. Efficacy of the Autoimmune Protocol Diet as Part of a Multi-disciplinary, Supported Lifestyle Intervention for Hashimoto’s Thyroiditis. Cureus. 2019;11(4):e4556. doi:10.7759/cureus.4556.
13.
Konijeti GG, Kim N, Lewis JD, Groven S, et al. Efficacy of the Autoimmune Protocol Diet for Inflammatory Bowel Disease. Inflamm Bowel Dis. 2017;23(11):2054–2060. doi:10.1097/MIB.0000000000001221.
14.
Ballantyne S. The Paleo Approach: Reverse Autoimmune Disease and Heal Your Body. Las Vegas, US: Victory Belt Publishing; 2014.
15.
Krishnamurthy HK, Reddy S, Jayaraman V, et al. Effect of Micronutrients on Thyroid Parameters. J Thyroid Res. 2021:1865483. doi:10.1155/2021/1865483.
16.
Chandrasekaran A, Groven S, Lewis JD, et al. An Autoimmune Protocol Diet Improves Patient-Reported Quality of Life in Inflammatory Bowel Disease. Crohns Colitis 360. 2019;1(3):otz019. doi:10.1093/crocol/otz019.
17.
Pałkowska-Goździk E, Lachowicz K, Rosołowska-Huszcz D. Effects of Dietary Protein on Thyroid Axis Activity. Nutrients. 2017;10(1):5. doi:10.3390/nu10010005.
18.
Dadej D, Szczepanek-Parulska E, Ruchała M. Interplay between Fatty Acid Binding Protein 4, Fetuin-A, Retinol Binding Protein 4 and Thyroid Function in Metabolic Dysregulation. Metabolites. 2022;12(4):300. https://doi.org/10.3390/metabo....
19.
Ott J, Promberger R, Kober F, et al. Hashimoto’s thyroiditis affects symptom load and quality of life unrelated to hypothyroidism: a prospective case-control study in women undergoing thyroidectomy for benign goiter. Thyroid. 2011;21(2):161–7. doi:10.1089/thy.2010.0191.
20.
Prummel MF, Wiersinga WM. Thyroid peroxidase autoantibodies in euthyroid subjects. Best Pract Res Clin Endocrinol Metab. 2005;19(1):1–15. doi: 10.1016/j.beem.2004.11.003.
21.
Smith MR, Fernandes J, Go YM, et al. Redox dynamics of manganese as a mitochondrial life-death switch. Biochem Biophys Res Commun. 2017;482(3):388–398. doi: 10.1016/j.bbrc.2016.10.126.
22.
Moncayo R, Moncayo H. The WOMED model of benign thyroid disease: Acquired magnesium deficiency due to physical and psychological stressors relates to dysfunction of oxidative phosphorylation. BBA Clin. 2014;3:44–64. doi:10.1016/j.bbacli.2014.11.002.
23.
Zoeller RT, Tan SW, Tyl RW. General background on the hypothalamic-pituitary-thyroid (HPT) axis. Crit Rev Toxicol. 2007;37(1–2):11–53. doi:10.1080/10408440601123446.
24.
Babić Leko M, Gunjača I, Pleić N, et al. Environmental Factors Affecting Thyroid-Stimulating Hormone and Thyroid Hormone Levels. Int J Mol Sci. 2021;22(12):6521. https://doi.org/10.3390/ijms22....
25.
Ventura M, Melo M, Carrilho F. Selenium and Thyroid Disease: From Pathophysiology to Treatment. Int J Endocrinol. 2017;2017:1297658. doi.org/1297658.10.1155/2017/1297658.
26.
Chung HR. Iodine and thyroid function. Ann Pediatr Endocrinol Metab. 2014;19(1):8–12. doi:10.6065/apem.2014.19.1.8.
27.
Pałkowska-Goździk E, Lachowicz K, Rosołowska-Huszcz D. Effects of Dietary Protein on Thyroid Axis Activity. Nutrients. 2018;10(1):5. doi: 10.3390/nu10010005.
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.