Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
RESEARCH PAPER
Effectiveness of sanitation regime in a milking parlour to control microbial contamination of teats and surfaces teat cups’
1
University of Veterinary Medicine and Pharmacy, Košice, Slovak Republic
Corresponding author
František Zigo
University of Veterinary Medicine and Pharmacy in Košice, UVLF, Komenského 73, Košice, Slovakia, 04001, Košice, Slovak Republic
University of Veterinary Medicine and Pharmacy in Košice, UVLF, Komenského 73, Košice, Slovakia, 04001, Košice, Slovak Republic
Ann Agric Environ Med. 2023;30(1):55-60
KEYWORDS
TOPICS
ABSTRACT
Introduction and objective:
The major sources of bacterial contamination of raw milk are post-harvest manipulation; therefore the disinfection of teat and teat cups which decrease the bacterial load has a positive impact on minimizing new infection rates. The aim of the study was determination of the incidence of pathogens on investigated surfaces, evaluation of the effectiveness of sanitation regime in the reduction of surface microbial load, and determination of the effectiveness of mechanical cleaning of teats in a milking parlour for dairy cows.
Material and methods:
Samples from surfaces were taken by microbiological swabs using a sterile cotton swab from area of 5×2 cm2. Sanitation regime was evaluated based on the effectiveness of active substances – lactic acid and sodium hypochlorite.
Results:
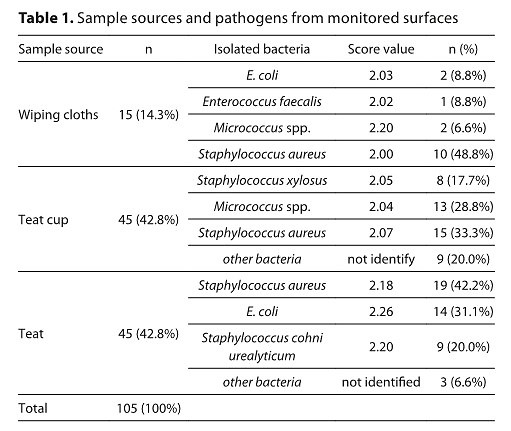
From a total of 105 swab, 44 samples were found positive for Staphylococcus aureus, 16 samples for E. coli, 15 samples for Micrococcus spp., 8 samples for Staphylococcus xylosus, 9 samples for Staphylococcus cohni urealyticum, 1 sample for Enterococcus faecalis. Among isolates, S. aureus was the predominat species from teats – 19/45, teat cups, 15/45 and from wiping cloths 10/15. Sanitation regime was confirmed by a decrease in the number of coliform bacteria (CB) determined on teat and teat cups from 2.33–0.95 Log10 CFU/cm2 (p<0.001) and 0.90–0.62 Log10 CFU/cm2 (p<0.001), respectively, and in the number of total bacteria count (TBC) determined on teat and teat cups from 4.36–0.99 Log10CFU/cm2 (p<0.001), and 1.85–0.77 Log10 CFU/cm2 (p<0.001), respectively. Incidence of CB (2.53 Log10 CFU/cm2) and TBC (3.83 Log10 CFU/cm2) on wiping cloths after mechanical cleaning of udders stress the importance of this step.
Conclusions:
Results show that disinfectant with lactic acid as the main active ingredient is suitable for bacterial reduction. Post-milking disinfection of teat and teat cups reduces bacterial contamination and proves to be most effective against environmental bacteria.
The major sources of bacterial contamination of raw milk are post-harvest manipulation; therefore the disinfection of teat and teat cups which decrease the bacterial load has a positive impact on minimizing new infection rates. The aim of the study was determination of the incidence of pathogens on investigated surfaces, evaluation of the effectiveness of sanitation regime in the reduction of surface microbial load, and determination of the effectiveness of mechanical cleaning of teats in a milking parlour for dairy cows.
Material and methods:
Samples from surfaces were taken by microbiological swabs using a sterile cotton swab from area of 5×2 cm2. Sanitation regime was evaluated based on the effectiveness of active substances – lactic acid and sodium hypochlorite.
Results:
From a total of 105 swab, 44 samples were found positive for Staphylococcus aureus, 16 samples for E. coli, 15 samples for Micrococcus spp., 8 samples for Staphylococcus xylosus, 9 samples for Staphylococcus cohni urealyticum, 1 sample for Enterococcus faecalis. Among isolates, S. aureus was the predominat species from teats – 19/45, teat cups, 15/45 and from wiping cloths 10/15. Sanitation regime was confirmed by a decrease in the number of coliform bacteria (CB) determined on teat and teat cups from 2.33–0.95 Log10 CFU/cm2 (p<0.001) and 0.90–0.62 Log10 CFU/cm2 (p<0.001), respectively, and in the number of total bacteria count (TBC) determined on teat and teat cups from 4.36–0.99 Log10CFU/cm2 (p<0.001), and 1.85–0.77 Log10 CFU/cm2 (p<0.001), respectively. Incidence of CB (2.53 Log10 CFU/cm2) and TBC (3.83 Log10 CFU/cm2) on wiping cloths after mechanical cleaning of udders stress the importance of this step.
Conclusions:
Results show that disinfectant with lactic acid as the main active ingredient is suitable for bacterial reduction. Post-milking disinfection of teat and teat cups reduces bacterial contamination and proves to be most effective against environmental bacteria.
ACKNOWLEDGEMENTS
This work was supported by grants VEGA 1-0162-23:
Reduction of antibiotic use in dairy mastitis control programs
and SAIA 2022-05-15-001: The effect of udder pathogens
on the production and degree of oxidative stress in dairy cows.
REFERENCES (46)
1.
Reta MA, Bereda TW, Alemu AN. Bacterial contaminations of raw cow’s milk consumed at Jigjiga City of Somali Regional State, Eastern Ethiopia. Int J Food Contam. 2016;3(1):1–9. http://doi.org/10.1186/s40550-....
2.
Gleeson D, O’Brien B, Jordan K. The effect of using nonchlorine products for cleaning and sanitising milking equipment on bacterial numbers and residues in milk. Int J Dairy Technol. 2013;66:182–188. http://doi: \10.1111/1471-0307.120373.
3.
Ózsvári L, Ivanyos D. The use of teat disinfectants and milking machine cleaning products in commercial Holstein-Friesian farms. Front Vet Sci. 2022;9:956843. http://doi:10.3389/fvets.2022.....
4.
Moroni P, Nydam DV, Ospina PA, Scillieri-Smith JC, Virkler PD, Watters RD, Welcome FL, Zurakowski MJ, Ducharme NG, Yeager AE. 8 – Diseases of the Teats and Udder. Sci Direct. 2018;389–465. https://doi.org/10.1016/B978-0....
5.
Sampimon O, Barkema H, Berends I, Sol J, Lam T. Prevalence of intramammary infection in Dutch dairy herds. J Dairy Res. 2009;76(2):129–136. https://doi: 0.1017/S0022029908003762.
6.
Harris K. Validation of the use of organic acids and acidified sodium chlorite to reduce Escherichia coli O157 and Salmonella Typhimurium in beef trim and ground beef in a simulated processing environment. J Food Protect. 2006;69:1802–1807.
7.
Pangloli P, Dje Y, Ahmed O, Doane CA, Oliver SP, Draughon FA. Seasonal incidence and molecular characterization of Salmonella from dairy cows, calves, and farm environment. Foodborne Pathog Dis. 2008;5(1):87–96. http://doi.org/10.1089/fpd.200....
8.
Oliver SP, Boor KJ, Murphy SC, Murinda SE. Food safety hazards associated with consumption of raw milk. Foodborne Pathogens and Disease. 2009;6(7):793–806. doi: 10.1089/fpd.2009.0302.
9.
Morton JM, Penry JF, Malmo J, Mein GA. Premilking teat disinfection: Is it worthwhile in pasture-grazed dairy herds? J Dairy Sci. 2014;97:7525–7537.
10.
Fitzpatrick SR, Garvey M, Flynn J, O´Brien B, Gleeson D. Effect of Pre-Milking Teat Foam Disinfection on the Prevention of New Mastitis Rates in Early Lactation. Animals. 2021;11:1–14.
11.
Dufour S, Fréchette A, Barkema HW, Mussell A, Scholl DT. Invited review: effect of udder health management practices on herd somatic cell count. J Dairy Sci. 2011;94:563–79.
12.
Suriyasathaporn W, and Chupia V. Reduction in numbers of bacteria after pre-milking teat dipping in milking dairy cows. CMU. J Nat Sci. 2011;10:301–306.
13.
Zucali M, Bava L, Tamburini A, Brasca M, Vanoni L, Sandrucci A. Effects of season, milking routine and cow cleanliness on bacterial and somatic cell counts of bulk tank milk. J Dairy Res. 2011;78:436–441. http://doi.org/10.1017/S002202....
14.
Mahmmod YS, Klaas IC, Svennesen L, Pedersen K, Ingmer H. Communications of Staphylococcus aureus and non-aureus Staphylococcus species from bovine intramammary infections and teat apex colonization. J Dairy Sci. 2018;101(8):7322–7333.
15.
Svennesen L, Nielsen SS, Mahmmod YS, Krömer V, Pedersen K, Klaas IC. Association between teat skin colonization and intramammary infection with Staphylococcus aureus and Streptococcus agalactiae in herds with automatic milking systems. J Dairy Sci. 2019;102(1):629–639.
16.
Williamson J, Malcolm D. Smart approach to post-milking teat disinfection. Proceedings of the New Zealand Milk Quality Conference; 2012; New Zealand. Veterinary Association, New Zealand Elsevier; 2013.
17.
Adkins PRF, Dufour S, Spain JN, Calcutt MJ, Reilly TJ, Stewart GC, et al. Cross-sectional study to identify staphylococcal species isolated from teat and inguinal skin of different-aged dairy heifers. J Dairy Sci. 2018;101(4):3213–3225. http://doi.org/10.3168/jds.201....
18.
Doyle CJ, Gleeson D, O‘Toole PW, Cotter PD. Impacts of Seasonal Housing and Teat Preparation on Raw Milk Microbiota: a High-Throughput Sequencing Study. Appl Environ Microb. 2017;83(2). https://doi.org/10.1128/AEM.02....
19.
Verdier-Metz I, Gagne G, Bornes S, Monsallier F, Veisseire P, Delbes-Paus C, Montel MC. Cow teat skin, a potential source of diverse microbial populations for cheese production. Appl Environ Microbiol. 2012;78:326–333. https://doi:10.1128/AEM.06229-....
20.
Ruegg PL. A 100-Year Review: Mastitis Detection, Management, and Prevention. J Dairy Sci. 2017;100:10381–10397.
21.
Zadoks R, Fitzpatrick J. Changing Trends in Mastitis. Irish Vet J. 2009;62:59–70. https://doi.org/10.1186/2046-0....
22.
Lin H, Shavezipur M, Yousef A, Maleky F. Prediction of growth of Pseudomonas fluorescens in milk during storage under fluctuating temperature. J Dairy Sci. 2016;99(3):1822–1830. http://doi.org/10.3168/jds.201....
23.
Van Kessel JS, Karns JS, Wolfgang DR, Hovingh E, Jayarao BM, Van Tassell CP, et al. Environmental sampling to predict fecal prevalence of Salmonella in an intensively monitored dairy herd. J Food Protect. 2008;71(10):1967–1973.
24.
Marchand S, Block JD, Jonghe VD, Coorevits A, Heyndrickx M, Herman L. Biofilm Formation in Milk Production and Processing Environments; Influence on Milk Quality and Safety. The Institute of Food Technologist‘s Comprehensive Reviews in Food Science and Food Safety. 2012;11(2):133–147.
25.
Du B, Meng L, Liu H, Zheng N, Zhang Y, Guo X, Zhao S, Li F, Wang J. Impacts of Milking and Housing Environment on Milk Microbiota. Animals. 2020;10(12):2339.
26.
Olivier D, Moshoeshoe SL. Incidence of aerobic spoilage-and psychrotrophic bacteria in non-pasteurised and pasteurised bovine milk from Maseru. Medical Technology SA. 2012;26(2):22–27.
27.
ISO 6887-5:2010. Microbiology of food and animal feeding stuffs. Preparation of test samples, initial suspension and decimal dilutions for microbiological examination. Part 5. Specific rules for the preparation of milk and milk products.
28.
ISO 18593:2004. Microbiology of food and animal feeding stuffs: Horizontal methods for sampling techniques from surfaces using contact plates. International Standard Organisation.
29.
ISO 4832:2006. Microbiology of food and animal feeding stuffs: Horizontal method or the enumeration of coliforms. International Standard Organisation.
30.
ISO 6888-1:1999. Microbiology of food and animal feeding stuffs – Horizontal method for the enumeration of coagulase-positive staphylococci (Staphylococcus aureus and other species) – Part 1. Technique using Baird-Parker agar medium.
31.
ISO 21528-1:2017. Microbiology of the Food Chain – Horizontal Method for the Detection and Enumeration of Enterobacteriaceae – Part 1. Detection of Enterobacteriaceae.
32.
De Jong E, De Jong AS, Smidts-van den Berg N, Rentenaar RJ. Differentiation of Raoultella ornithinolytica/planticola and Klebsiella oxytoca clinical isolates by matrix-assisted laser desorption/ionization-time of flight mass spectrometry. Diagn Micr Infec Dis. 2013;75(4):431–433.
33.
Vijaya Kumar A, Venkateswara Rao L, Kishan Kumar M, Srinu B, Madhava Rao T. Efficacy of Udder Disinfectants on Reduction of Bacterial Load and Certain Pathogens of Public Health Significance. J Microbiol Biotechn Res. 2012;2:147–51.
34.
Breen J. The Importance of Teat Disinfection in Mastitis Control. Livest. 2019;24(3):122–128.
35.
Dufour S, Fréchette A, Barkema HW, Mussell A, Scholl DT. Invited review: effect of udder health management practices on herd somatic cell count. J Dairy Sci. 2011;94:563–579.
36.
Gleeson D, O’Brien B, Flynn J, O’Callaghan E, Galli F. Effect of pre-milking teat preparation procedures on the microbial count on teats prior to cluster application. Irish Vet J. 2009;62:461–467.
37.
Salustiano VC, Nelio JA, Sebastiao CB, Willian M, Gabriela P. An assessment of chemical sanitizers on the microbiological profile of air in a milk processing plant. J Food Saf. 2004;24:159–167.
38.
Derakhshani H, Fehr KB, Sepehri S, Francoz D, De Buck J, Barkema HW, Plaizier JC, Khafipour E. Microbiota of the bovine udder: Contributing factors and potential implications for udder health and mastitis susceptibility. J Dairy Sci. 2018;101(12):10605–10625.
39.
Baumberger C, Guarin JF, Ruegg PL. Effect of 2 different pre-milking teat sanitation routines on reduction of bacterial counts on teat skin of cows on commercial dairy farms. J Dairy Sci. 2016;99:2915–2929.
40.
Tamime AY. Milk Processing and Quality Management: Quality Control. Belleque J, Chicon R, Recio I, editors. West Sussex, UK: John Wiley and sons publishers; 2009. p. 56–60.
41.
Deak E, Charlton CL, Bobenchik AM, Miller SA, Pollett S, McHardy IH, Wu MT, Garner OB. Comparison of the Vitek MS and Bruker Microflex LT MALDI-TOF MS platforms for routine identification of commonly isolated bacteria and yeast in the clinical microbiology laboratory. Diagn Microbiol Infect Dis. 2015;81(1):27–33.
42.
Abril AG, Villa TG, Barros-Velázquez J, Canas B, Sánchez-Pérez A, Calo-Mata P, Carrera M. Staphylococcus aureus Exotoxins and Their Detection in the Dairy Industry and Mastitis. Toxins. 2020;12(9):537.
43.
Monistero V, Graber HU, Pollera C, Cremonesi P. Staphylococcus aureus Isolates from Bovine Mastitis in Eight Countries: Genotypes, Detection of Genes Encoding Different Toxins and Other Virulence Genes. Toxins. 2018;10(6):247–249.
44.
Mišeikiene R, Rudejeviene J, Gerulis G. Effect of Pre-Milking Antiseptic Treatment on the Bacterial Contamination of Cow Teats’ Skin. Bulg J Vet Med. 2015;18:159–166.
45.
Godden SM, Royster E, Knauer W, Sorg J, Lopez-Benavides M, Schukken Y, French EA. Randomized non-inferiority study evaluating the efficacy of a post-milking teat disinfectant for the prevention of naturally occurring intramammary infections. J Dairy Sci. 2016;99:3675–3687.
46.
Fitzpatrick SR, Garvey M, Flynn J, Jordan K, Gleeson D. Are Some Teat Disinfectant Formulations More Effective against Specific Bacteria Isolated on Teat Skin Than Others? Acta Vet Scan. 2019;61:1–5.
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.