Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
RESEARCH PAPER
Faecal Microbiota Transfer – a new concept for treating cytomegalovirus colitis in children with
ulcerative colitis
1
Department of Paediatric Gastroenterology and Nutrition, Medical University, Warsaw, Poland
2
School of Public Health, Centre of Postgraduate Medical Education, Preventive Medicine and Rehabilitation Centre, Foundation for Infection Prevention Institute, Warsaw, Poland
Corresponding author
Izabella Lazowska-Przeorek
Department of Pediatric Gastroenterology and Nutrition, Medical University of Warsaw, Żwirki i Wigury 63 a, 02-091, Warsaw, Poland
Department of Pediatric Gastroenterology and Nutrition, Medical University of Warsaw, Żwirki i Wigury 63 a, 02-091, Warsaw, Poland
Ann Agric Environ Med. 2021;28(1):56-60
KEYWORDS
TOPICS
ABSTRACT
Introduction:
Cytomegalovirus (CMV) infection in patients with inflammatory bowel disease (IBD) is reactivated by the use of immunosuppressive drugs. CMV infection may produce IBD flares refractory to standard therapy.
Objective:
The aim of our study was to assess the efficacy and safety of faecal microbiota transplantation (FMT) for the treatment of CMV colitis in patients with ulcerative colitis (UC) flare.
Material and methods:
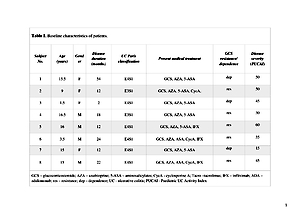
A total of 8 children, with mild to severe UC, positive for CMV PCR in colonic biopsies, received 50–100 ml FMT by nasogastric tube on 5 consecutive days in each of 2 weeks. During the study, the subjects were treated with 5ASA and FMT. Immunosuppressant therapy was withdrawn, when CMV colitis was diagnosed by positive DNA PCR in colonic tissues. The clinical response was defined as a decrease of Paediatric UC Activity Index by ≥20 points.
Results:
At the 6th week of the study, negative colonic CMV DNA PCR was measured after 10 infusions in 7/8 patients. For one boy, 20 infusions were administered to assess CMV elimination from colonic biopsies. A clinical response was observed in 3/8 patients, with clinical remission in 3/8 patients. Faecal calprotectin decreased significantly in 3 patients. CRP normalized in 2 patients after 6 weeks. No serious adverse effects were observed during and after infusions.
Conclusions:
FMT seems to be an effective and safe treatment option for CMV colitis in children with UC. This is the first study to demonstrate the application of FMT as a new therapeutic option for CMV colitis.
Cytomegalovirus (CMV) infection in patients with inflammatory bowel disease (IBD) is reactivated by the use of immunosuppressive drugs. CMV infection may produce IBD flares refractory to standard therapy.
Objective:
The aim of our study was to assess the efficacy and safety of faecal microbiota transplantation (FMT) for the treatment of CMV colitis in patients with ulcerative colitis (UC) flare.
Material and methods:
A total of 8 children, with mild to severe UC, positive for CMV PCR in colonic biopsies, received 50–100 ml FMT by nasogastric tube on 5 consecutive days in each of 2 weeks. During the study, the subjects were treated with 5ASA and FMT. Immunosuppressant therapy was withdrawn, when CMV colitis was diagnosed by positive DNA PCR in colonic tissues. The clinical response was defined as a decrease of Paediatric UC Activity Index by ≥20 points.
Results:
At the 6th week of the study, negative colonic CMV DNA PCR was measured after 10 infusions in 7/8 patients. For one boy, 20 infusions were administered to assess CMV elimination from colonic biopsies. A clinical response was observed in 3/8 patients, with clinical remission in 3/8 patients. Faecal calprotectin decreased significantly in 3 patients. CRP normalized in 2 patients after 6 weeks. No serious adverse effects were observed during and after infusions.
Conclusions:
FMT seems to be an effective and safe treatment option for CMV colitis in children with UC. This is the first study to demonstrate the application of FMT as a new therapeutic option for CMV colitis.
REFERENCES (26)
1.
Shukla T, Singh S, Tandon P, McCurdy J. Corticosteroids and thiopurines, but not Tumor Necrosis Factor Antagonist, associated with Cytomegalovirus reactivation in Inflammatory Bowel Disease. A systemic review and meta-analysis. J Clin Gastreonerol. 2017; 51: 394–401.
2.
Sager K, Alam S, Bond A, Chinnappan L, Probert C S. Review article: cytomegalovirus and inflammatory bowel disease. Aliment Pharmacol Ther. 2015; 41: 725–733.
3.
Bontà J, Zeitz J, Frei P, Biedermann L, Sulz M C, Vavricka S R, et.al. Cytomegalovirus disease in inflammatory bowel disease: epidemiology and disease characteristics in a large single-centre experience. Eur J Gastroenterol Hepatol. 2016; 28: 1329–1334.
4.
Zagórowicz E, Bugajski M, Wieszczy P, Pietrzak A, Magdziak A, Mróz A. Cytomegalovirus infection in ulcerative colitis is related to severe inflammation and a high count of cytomegalovirus-positive cells in biopsy is a risk factor for colectomy. J Crohn’s Colitis. 2016; 10: 1205–1211.
5.
Kishore J, Ghoshal U, Ghoshal U C, Krishnani N, Kumar S, Singh M, et.al. Infection with cytomegalovirus in patients with inflammatory bowel disease: prevalence, clinical significance and outcome. J Med Microbiol. 2004; 53: 1155–1160.
6.
Rahier J F, Magro F, Abreu C, Armuzzi A, Ben-Horin S, Chowers Y, et.al. European Crohn’s and Colitis Organisation (ECCO). Second European evidence-based consensus on the prevention, diagnosis and management of opportunistic infections in inflammatory bowel disease. J Crohns Colitis. 2014; 8: 443–468.
7.
Pillet S, Pozzetto B, Jarlot C, Paul S, Roblin X. Management of cytomegalovirus infection in inflammatory bowel diseases. Dig Liver Dis. 2012; 44: 541–548.
8.
Pillet S, Pozzetto B, Roblin X. Cytomegalovirus and ulcerative colitis: Place of antiviral therapy. World J Gastroenterol. 2016; 22: 2030–2045.
9.
Gwee A, Curtis N, Connell T G, Garland S, Daley A J. Ganciclovir for the treatment of congenital cytomegalovirus: what are the side effects? Pediatr Infect Dis J. 2014; 33: 115.
10.
Fukuchi T, Nakase H, Matsuura M, Yoshino T, Toyonaga T, Ohmori K, et. al. Effect of intensive granulocyte and monocyte adsorptive apheresis in patients with ulcerative colitis positive for cytomegalovirus. J Crohns Colitis. 2013; 7: 803–811.
11.
Yoshino T, Nakase H, Matsuura M, Matsumura K, Honzawa Y, Fukuchi T, et.al. Effect and safety of granulocyte-monocyte adsorption apheresis for patients with ulcerative colitis positive for cytomegalovirus in comparison with immunosuppressants. Digestion. 2011; 84: 3–9.
12.
Moayyedi P, Surette M G, Kim P T, Libertucci J, Wolfe M, Onischi C, et. al. Fecal microbiota transplantation induces remission in patients with active ulcerative colitis in a randomized, controlled trial. Gastroenterology. 2015; 149: 102–109.
13.
Levine A, Koletzko S, Turner D, Escher J C, Cucchiara S, de Ridder L, et.al. European Society of Pediatric Gastroenterology, Hepatology, and Nutrition. ESPGHAN revised porto criteria for the diagnosis of inflammatory bowel disease in children and adolescents. J Pediatr Gastroenterol Nutr. 2014; 58: 795–806.
14.
Hourigan S K, Chen L A, Grigoryan Z, Laroche G, Weidner M, Sears C L, et al. Microbiome changes associated with sustained eradication of Clostridium difficile after single faecal microbiota transplantation in children with and without inflammatory bowel disease. Aliment Pharmacol Ther. 2015; 42: 741–752.
15.
Russell G H, Kaplan J L, Youngster I, Baril-Dore M, Schindelar L, Hohmann E, et al. Fecal transplant for recurrent Clostridium difficile infection in children with and without inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 2014; 58: 588–592.
16.
Meighani A, Hart B R, Bourgi K, Miller N, John A, Ramesh M. Outcomes of fecal microbiota transplantation for Clostridium difficile infection in patients with inflammatory bowel disease. Dig Dis Sci. 2017; 62: 2870–2875.
17.
Khoruts A, Rank K M, Newman K M, Viskocil K, Vaughn B P, Hamilton M J, et al. Inflammatory bowel disease affects the outcome of fecal microbiota transplantation for recurrent Clostridium difficile infection. Clin Gastroenterol Hepatol. 2016; 14: 1433–1438.
18.
Kelly C R, Ihunnah C, Fischer M, Khoruts A, Surawicz C, Afzali A, et al. Fecal microbiota transplant for treatment of Clostridium difficile infection in immunocompromised patients. Am J Gastroenterol. 2014; 109: 1065–1071.
19.
Kunde S, Pham A, Bonczyk S, Crumb T, Duba M, Conrad H Jr, et al. Safety, tolerability and clinical response after fecal microbiota transplantation in children and young adults with ulcerative colitis. J Pediatr Gastroenterol Nutr. 2013; 56: 597–601.
20.
Suskind D L, Singh N, Nielson H, Wahbeh G. Fecal microbiota transplantation via nasogastric tube for active pediatric ulcerative colitis. J Pediatr Gastroenterol Nutr. 2015; 60: 27–29.
21.
Suskind D L, Brittnacher M J, Wahbeh G, Shaffer M L, Hayden H S, Qin X, et al. Fecal microbial transplant effect on clinical outcomes and fecal microbiome in active Crohn’s disease. Inflamm Bowel Dis. 2015; 21: 556–563.
22.
Kellermayer R, Nagy-Szakal D, Harris R A, Luna R A, Pitashny M, Schady D, et al. Serial fecal microbiota transplantation alters mucosal gene expression In pediatric ulcerative colitis. Am J Gastroenterol. 2015; 110: 604–606.
23.
Rossen N G, Fuentes S, van der Spek M J, Tijssen J G, Hartman J H, Duflou A, et al. Findings from a randomized controlled trial of fecal transplantation for patients with ulcerative colitis. Gastroenterology. 2015; 149: 110–118.
24.
Yoshino T, Nakase H, Ueno S, Uza N, Inoue S, Mikami S, et al. Usefulness of quantitative real-time PCR assay for early detection of cytomegalovirus infection in patients with ulcerative colitis refractory to immunosuppressive therapies. Inflamm Bowel Dis. 2007; 13: 1516–1521.
25.
Thörn M, Rorsman F, Rönnblom A, Sangfelt P, Wanders A, Eriksson B M, et al. Active cytomegalovirus infection diagnosed by real-time PCR in patients with inflammatory bowel disease: a prospective, controlled observational study. Scand J Gastroenterol. 2016; 51: 1075–1080.
26.
Roblin X, Pillet S, Oussalah A, Berthelot P, Del Tedesco E, Phelip J M, et al. Cytomegalovirus load in inflamed intestinal tissue is predictive of resistance to immunosuppressive therapy in ulcerative colitis. Am J Gastroenterol. 2011; 106: 2001–2008.
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.