Online first
Current issue
Archive
Special Issues
About the Journal
Publication Ethics
Anti-Plagiarism system
Instructions for Authors
Instructions for Reviewers
Editorial Board
Editorial Office
Contact
Reviewers
All Reviewers
2025
2024
2023
2022
2021
2020
2019
2018
2017
2016
General Data Protection Regulation (RODO)
RESEARCH PAPER
The development of cigarette smoke induced chronic pancreatitis in mice is associated with increased expression of K-Ras and NF-κB
1
Department of Gastroenterology and Internal Medicine, Medical University, Bialystok, Poland
2
Department of Paediatrics, Gastroenterology, Hepatology, Nutrition and Allergology, Medical University, Bialystok, Poland
3
Department of Pathomorphology, Medical University, Bialystok, Poland
4
Department of Medical Biology, Medical University, Bialystok, Poland
5
Department of Hygiene, Epidemiology and Metabolic Disorders, Medical University, Białystok, Poland
Corresponding author
Jaroslaw Daniluk
Department of Gastroenterology and Internal Medicine, Medical University of Bialystok, M.Składowskiej-Curie 24a, 15-276, Białystok, Poland
Department of Gastroenterology and Internal Medicine, Medical University of Bialystok, M.Składowskiej-Curie 24a, 15-276, Białystok, Poland
Ann Agric Environ Med. 2022;29(2):246-251
KEYWORDS
TOPICS
- Biological agents posing occupational risk in agriculture, forestry, food industry and wood industry and diseases caused by these agents (zoonoses, allergic and immunotoxic diseases)
- State of the health of rural communities depending on various factors: social factors, accessibility of medical care, etc.
ABSTRACT
Introduction and objective:
Epidemiological studies have demonstrated a strong association between cigarette smoking (CS) and chronic pancreatitis (CP); however, the exact mechanisms of this phenomenon remains unknown. The authors have previously shown that increased Ras expression activates the NF-κB mediated pathway and promotes development of CP. However, it is unclear whether a similar phenomenon occurs in CS-induced CP. Therefore, the aim of the study was to determine whether CS increases the expression of K-Ras, and promotes the development of CP in mice exposed to repeated episodes of acute pancreatitis (AP).
Material and methods:
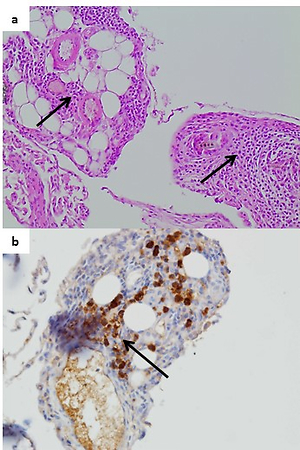
C57BL6/cmdb mice were exposed to CS or a sham treatment for 12 weeks. After one week of exposure, half of the animals from both groups were additionally subjected to repeated cerulein treatment (once a week, for 10 consecutive weeks) to mimic recurrent episodes of AP. Extension of pancreatic damage was determined histologically by H&E and Trichrome staining. The expression of K-Ras protein and downstream components (NF-κB, Cox-2, TGF-β) was evaluated by immunohistochemistry.
Results:
C57BL6/cmdb mice exposed to CS or cerulein alone did not develop any chronic pancreatic damage. However, concomitant treatment with both of these agents caused focal acinar atrophy, with slight intralobular and perivascular areas of fibrosis, and inflammatory cells infiltration resembling mild CP. Moreover, immunohistochemistry examinations revealed increased pancreatic expression of K-Ras and NF-κB only in mice treated both with CS and cerulein.
Conclusions:
CS promotes development of CP in mice exposed to repeated episodes of AP. This process may be, at least partially, related to increased expression of K-Ras and NF-κB protein.
Epidemiological studies have demonstrated a strong association between cigarette smoking (CS) and chronic pancreatitis (CP); however, the exact mechanisms of this phenomenon remains unknown. The authors have previously shown that increased Ras expression activates the NF-κB mediated pathway and promotes development of CP. However, it is unclear whether a similar phenomenon occurs in CS-induced CP. Therefore, the aim of the study was to determine whether CS increases the expression of K-Ras, and promotes the development of CP in mice exposed to repeated episodes of acute pancreatitis (AP).
Material and methods:
C57BL6/cmdb mice were exposed to CS or a sham treatment for 12 weeks. After one week of exposure, half of the animals from both groups were additionally subjected to repeated cerulein treatment (once a week, for 10 consecutive weeks) to mimic recurrent episodes of AP. Extension of pancreatic damage was determined histologically by H&E and Trichrome staining. The expression of K-Ras protein and downstream components (NF-κB, Cox-2, TGF-β) was evaluated by immunohistochemistry.
Results:
C57BL6/cmdb mice exposed to CS or cerulein alone did not develop any chronic pancreatic damage. However, concomitant treatment with both of these agents caused focal acinar atrophy, with slight intralobular and perivascular areas of fibrosis, and inflammatory cells infiltration resembling mild CP. Moreover, immunohistochemistry examinations revealed increased pancreatic expression of K-Ras and NF-κB only in mice treated both with CS and cerulein.
Conclusions:
CS promotes development of CP in mice exposed to repeated episodes of AP. This process may be, at least partially, related to increased expression of K-Ras and NF-κB protein.
REFERENCES (25)
1.
Beyer G, Habtezion A, Werner J, Lerch MM, Mayerle J. Chronic pancreatitis. Lancet. 2020;396:499–512. https://doi.org/10.1016/S0140–....
2.
Kirkegard J, Mortensen FV, Cronin-Fenton D. Chronic Pancreatitis and Pancreatic Cancer Risk: A Systematic Review and Meta-analysis. Am J Gastroenterol. 2017;112:1366–72 https://doi.org/10.1038/ajg.20....
3.
Olesen SS, Mortensen LH, Zinck E, Becker U, Drewes AM, Nojgaard C, et al. Time trends in incidence and prevalence of chronic pancreatitis: A 25-year population-based nationwide study. United European Gastroenterol J. 2021;9:82–90. https://doi.org/10.1177/205064....
4.
Jeon CY, Feldman R, Althouse A, AlKaade S, Brand RE, Guda N, et al. Lifetime smoking history and cohort-based smoking prevalence in chronic pancreatitis. Pancreatology. 2021. Online ahead of print https://doi.org/10.1016/j.pan.....
5.
Weiss FU, Laemmerhirt F, Lerch MM. Etiology and Risk Factors of Acute and Chronic Pancreatitis. Visc Med. 2019;35:73–81. https://doi.org/10.1159/000499....
6.
Edderkaoui M, Thrower E. Smoking and Pancreatic Disease. J Cancer Ther. 2013;4:34–40. https://doi.org/10.4236/jct.20....
7.
Ye X, Lu G, Huai J, Ding J. Impact of smoking on the risk of pancreatitis: a systematic review and meta-analysis. PLoS One. 2015;10:e0124075. https://doi.org/10.1371/journa....
8.
Setiawan VW, Pandol SJ, Porcel J, Wilkens LR, Le Marchand L, Pike MC, et al. Prospective Study of Alcohol Drinking, Smoking, and Pancreatitis: The Multiethnic Cohort. Pancreas. 2016;45:819–25. https://doi.org/10.1097/MPA.00....
9.
Hegyi P, Párniczky A, Lerch MM, Sheel ARG, Rebours V, Forsmark CE, et al. International Consensus Guidelines for Risk Factors in Chronic Pancreatitis. Recommendations from the working group for the international consensus guidelines for chronic pancreatitis in collaboration with the International Association of Pancreatology, the American Pancreatic Association, the Japan Pancreas Society, and European Pancreatic Club. Pancreatology. 2020;20:579–85. https://doi.org/10.1016/j.pan.....
10.
Prokopczyk B, Hoffmann D, Bologna M, Cunningham AJ, Trushin N, Akerkar S, et al. Identification of tobacco-derived compounds in human pancreatic juice. Chem Res Toxicol. 2002;15:677–85. https://doi.org/10.1021/tx0101....
11.
Chowdhury P, Udupa KB. Effect of nicotine on exocytotic pancreatic secretory response: role of calcium signaling. Tob Induc Dis. 2013;11:1. https://doi.org/10.1186/1617–9....
12.
Li Z, Lu D, Jin T, Liu X, Hao J. Nicotine facilitates pancreatic fibrosis by promoting activation of pancreatic stellate cells via ?7nAChR-mediated JAK2/STAT3 signaling pathway in rats. Toxicol Lett. 2021;349:84–91. https://doi.org/10.1016/j.toxl....
13.
Hermann PC, Sancho P, Canamero M, Martinelli P, Madriles F, Michl P, et al. Nicotine promotes initiation and progression of KRAS-induced pancreatic cancer via Gata6-dependent dedifferentiation of acinar cells in mice. Gastroenterology. 2014;147:1119–33.e4. https://doi.org/10.1053/j.gast....
14.
Wittel UA, Singh AP, Henley BJ, Andrianifahanana M, Akhter MP, Cullen DM, et al. Cigarette smoke-induced differential expression of the genes involved in exocrine function of the rat pancreas. Pancreas. 2006;33:364–70. https://doi.org/10.1097/01.mpa....
15.
Xue J, Zhao Q, Sharma V, Nguyen LP, Lee YN, Pham KL, et al. Aryl Hydrocarbon Receptor Ligands in Cigarette Smoke Induce Production of Interleukin-22 to Promote Pancreatic Fibrosis in Models of Chronic Pancreatitis. Gastroenterology. 2016;151:1206–17. https://doi.org/10.1053/j.gast....
16.
Daniluk J, Liu Y, Deng D, Chu J, Huang H, Gaiser S, et al. An NF-?B pathway-mediated positive feedback loop amplifies Ras activity to pathological levels in mice. J Clin Invest. 2012;122:1519–28. https://doi.org/10.1172/JCI597....
17.
Philip B, Roland CL, Daniluk J, Liu Y, Chatterjee D, Gomez SB, et al. A high-fat diet activates oncogenic Kras and COX2 to induce development of pancreatic ductal adenocarcinoma in mice. Gastroenterology. 2013;145:1449–58. https://doi.org/10.1053/j.gast....
18.
Huang H, Daniluk J, Liu Y, Chu J, Li Z, Ji B, et al. Oncogenic K-Ras requires activation for enhanced activity. Oncogene. 2014;33:532–5. https://doi.org/10.1038/onc.20....
19.
Xiao AY, Tan ML, Wu LM, Asrani VM, Windsor JA, Yadav D, et al. Global incidence and mortality of pancreatic diseases: a systematic review, meta-analysis, and meta-regression of population-based cohort studies. Lancet Gastroenterol Hepatol. 2016;1:45–55. https://doi.org/10.1016/S2468–....
20.
Wittel UA, Pandey KK, Andrianifahanana M, Johansson SL, Cullen DM, Akhter MP, et al. Chronic pancreatic inflammation induced by environmental tobacco smoke inhalation in rats. Am J Gastroenterol. 2006;101:148–59. https://doi.org/10.1111/j.1572....
21.
Huang H, Swidnicka-Siergiejko AK, Daniluk J, Gaiser S, Yao Y, Peng L, et al. Transgenic Expression of PRSS1. Gastroenterology. 2020;158:1072–82.e7. https://doi.org/10.1053/j.gast....
22.
Haritha J, Wilcox CM. Evaluation of Patients’ Knowledge Regarding Smoking and Chronic Pancreatitis: A Pilot Study. J Gastroenterol Pancreatol Liver Disord. 2015;1:1–4.
23.
Lindkvist B, Wierup N, Sundler F, Borgström A. Long-term nicotine exposure causes increased concentrations of trypsinogens and amylase in pancreatic extracts in the rat. Pancreas. 2008;37:288–94. https://doi.org/10.1097/MPA.0b....
24.
Kumar S, Torres MP, Kaur S, Rachagani S, Joshi S, Johansson SL, et al. Smoking accelerates pancreatic cancer progression by promoting differentiation of MDSCs and inducing HB-EGF expression in macrophages. Oncogene. 2015;34:2052–60. https://doi.org/10.1038/onc.20....
25.
Porta M, Crous-Bou M, Wark PA, Vineis P, Real FX, Malats N, et al. Cigarette smoking and K-ras mutations in pancreas, lung and colorectal adenocarcinomas: etiopathogenic similarities, differences and paradoxes. Mutat Res. 2009;682:83–93. https://doi.org/10.1016/j.mrre....
Share
RELATED ARTICLE
We process personal data collected when visiting the website. The function of obtaining information about users and their behavior is carried out by voluntarily entered information in forms and saving cookies in end devices. Data, including cookies, are used to provide services, improve the user experience and to analyze the traffic in accordance with the Privacy policy. Data are also collected and processed by Google Analytics tool (more).
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.
You can change cookies settings in your browser. Restricted use of cookies in the browser configuration may affect some functionalities of the website.